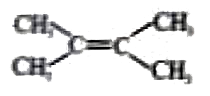
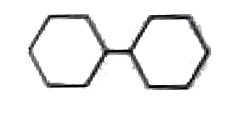
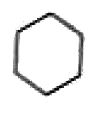
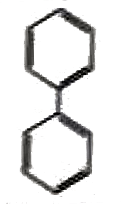A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PHYSICS WALLAH-CHEMICAL BONDING AND MOLECULAR STRUCTURE -NEET Past 5 Years Questions
- Predict the correct order omong the following:
Text Solution
|
- Which of the following set of molecules will have zero dipole moment?
Text Solution
|
- Identify a molecule which does not exist.
Text Solution
|
- How many sp^2 hybridised carbon atoms and pi bonds respectively are pr...
Text Solution
|
- The potential energy (y) curve for H2 formation as a function of inte...
Text Solution
|
- Identify the wrongly matched pair.
Text Solution
|
- Which of the following diatomic molecular species has only bonds accor...
Text Solution
|
- Identify the incorrect statement related to PCI5 from the following:
Text Solution
|
- Consider the following species CN^(-),CN^(-),NO and CN. Which one ...
Text Solution
|
- Which one of the following pair of species have the same bond order?
Text Solution
|
- The species, having bonds angle of 120^(@) is
Text Solution
|
- which one of the following ions is not tetrahedral in shape ?
Text Solution
|
- Which of the following hydrides has the smallest bond angle?
Text Solution
|
- Which one of the following compounds shows the presence of intramolecu...
Text Solution
|
- In which of the following molecules, all atoms are coplanar?
Text Solution
|
- Among the following which one is a wrong statement?
Text Solution
|
- The hybridisations of atomic orbitals of nitrogen in NO2, NO3^(-) and ...
Text Solution
|
- Which of the following pairs of ions is isoelectronic and isostructur...
Text Solution
|
- Consider the molecules CH(4),NH(3) and H(2)O which of the given statem...
Text Solution
|
- Predict the correct order omong the following:
Text Solution
|