Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR-ACIDS , BASES AND SALTS -SHORT ANSWER QUESTIONS
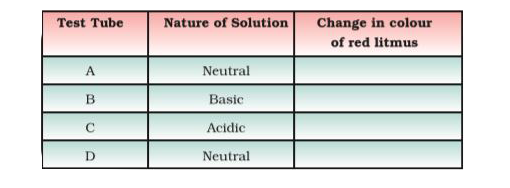
- Look at Figure 5.1 which shows solutions taken in test tubes A,B,...
Text Solution
|
- While playing in a park , a child was stung by a wasp . Some elders s...
Text Solution
|
- Form a sentence using the following words - baking soda, ant bite, m...
Text Solution
|
- Match the substance in Column I with those in Column II .
Text Solution
|
- Fill the blanks in the following sentences Lemon juice and vine...
Text Solution
|
- Turmeric and litmus are acid-base indicators.
Text Solution
|
- Phenolphthalein gives colour with lime water.
Text Solution
|
- When an acidic solution is mixed with a basic solution, they each...
Text Solution
|