Text Solution
Verified by Experts
Topper's Solved these Questions
ARYL DIAZONIUM SALTS
ARIHANT PUBLICATION|Exercise QUESTIONS FOR PRACTICE (SHORT ANSWER TYPE I QUESTIONS 2 MARK) |4 VideosARYL DIAZONIUM SALTS
ARIHANT PUBLICATION|Exercise QUESTIONS FOR PRACTICE (SHORT ANSWER TYPE II QUESTIONS 3 MARK) |5 VideosARYL DIAZONIUM SALTS
ARIHANT PUBLICATION|Exercise CHAPTER PRACTICE (LONG ANSWER TYPE QUESTIONS) 7 MARKS |2 VideosAMINES
ARIHANT PUBLICATION|Exercise Chapter Practice (Long Answer Type Questions)|2 VideosCARBOXYLIC ACIDS
ARIHANT PUBLICATION|Exercise CHAPTER PRACTICE (LONG ANSWER TYPE QUESTIONS) |3 Videos
Similar Questions
Explore conceptually related problems
ARIHANT PUBLICATION-ARYL DIAZONIUM SALTS -QUESTIONS FOR PRACTICE (VERY SHORT ANSWER TYPE QUESTIONS 1 MARK)
- Write the structure of benzene diazonium chloride.
Text Solution
|
- The conversion of primary aromatic amines into diazonium salts is know...
Text Solution
|
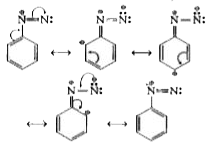
- Diazonium salts of aromatic amines are more stable than those of aliph...
Text Solution
|
- Why is benzene diazonium chloride not stored and used immediately afte...
Text Solution
|
- Complete the following reactions. (i) C(6)H(5) N(2)Cl + H(3)PO(2) + ...
Text Solution
|
- What is Sandmeyer's reaction ?
Text Solution
|
- Complete the following reaction: C(6)H(5)N(2)Cl + C(2)H(5)OH to
Text Solution
|
- Benzene diazonium chloride reacts with potassium iodide to form
Text Solution
|
- Complete the following reaction. C(6)H(5)N(2)^(+)Cl^(-) overset(H(2)...
Text Solution
|
- Write the chemical reaction occuring in the preparation of fluorobenze...
Text Solution
|
- What do you mean by coupling reaction?
Text Solution
|
- Benzene diazonium chloride reacts with phenol inmedium to form an oran...
Text Solution
|
- Which compound is formed when benzene diazonium chloride reacts with p...
Text Solution
|