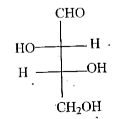
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
STEREOCHEMISTRY
BRILLIANT PUBLICATION|Exercise LEVEL-II (ASSERTION - REASON TYPE)|2 VideosSTEREOCHEMISTRY
BRILLIANT PUBLICATION|Exercise LEVEL-II (ASSERTION - REASON TYPE)|2 VideosSOLUTIONS
BRILLIANT PUBLICATION|Exercise Level-III (Linked Comprehension Type)|12 VideosSURFACE CHEMISTRY
BRILLIANT PUBLICATION|Exercise QUESTIONS (LEVEL - II) (ASSERTION REASON TYPE QUESTIONS) |6 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-STEREOCHEMISTRY -LEVEL-II
- The most stable conformer of meso-1,2-dibromo-1, 2-dichloro ethane is
Text Solution
|
- In the dehydrohalogenatino of 2-bromobutane, which conformation leads ...
Text Solution
|
- How many geometrical isomers are possible for the following structure
Text Solution
|
- Which of the following exhibit geometrical isomerism
Text Solution
|
- Which of the following is E-configuration
Text Solution
|
- The IUPAC name of the compound
Text Solution
|
- Minimum number of carbonatoms for a substituted alkene to show geometr...
Text Solution
|
- The transformation of (+) or (-) form to racemic mixture (pm) is calle...
Text Solution
|
- How many chiral centres are present in the following molecules
Text Solution
|
- The following two compounds are
Text Solution
|
- (+) Mandelic acid has a specific rotation of +158^@. What would be the...
Text Solution
|
- The optically active and meso forms possible for the following structu...
Text Solution
|
- Methyl ethyl amine cannot be resolved into enantiomers because :
Text Solution
|
- Select the optically inactive compound among the following
Text Solution
|
- In the following reaction the most probable product will be
Text Solution
|
- Assign R or S configuration to the Fischer projection of the following...
Text Solution
|
- What are the configurations of carbon atoms 2 and 3 respectively in D-...
Text Solution
|
- During debromination of meso-2, 3-dibromo butane the major compound fo...
Text Solution
|
- On monochlorination of 2-methyl butane, the total number of chiral com...
Text Solution
|