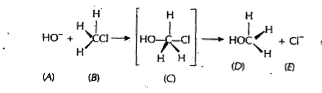
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Assertion: In monohaloarenes, further electrophilic substitution occur...
Text Solution
|
- Halogens are deactivating yet ortho,para directing in electrophilic ...
Text Solution
|
- Assertion : Nitration of benzoic acid gives meta nitrobenzoic acid. Re...
Text Solution
|
- Assertion: In monohaloarenes, further electrophilic substitution occur...
Text Solution
|
- Assertion: In case of aryl halides, halogens are moderately deactivati...
Text Solution
|
- Assertion: In monohaloarenes, further electrophilic substitution occur...
Text Solution
|
- Assertion: IN mono haloarenes, electrophilic substitution occurs at or...
Text Solution
|
- Assertion (A) : In monohaloarenes, further electrophilic substitution ...
Text Solution
|
- In ary halides halogen group is a ortho para director and a deactivato...
Text Solution
|