A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN -TEST PAPERS-PHYSICS
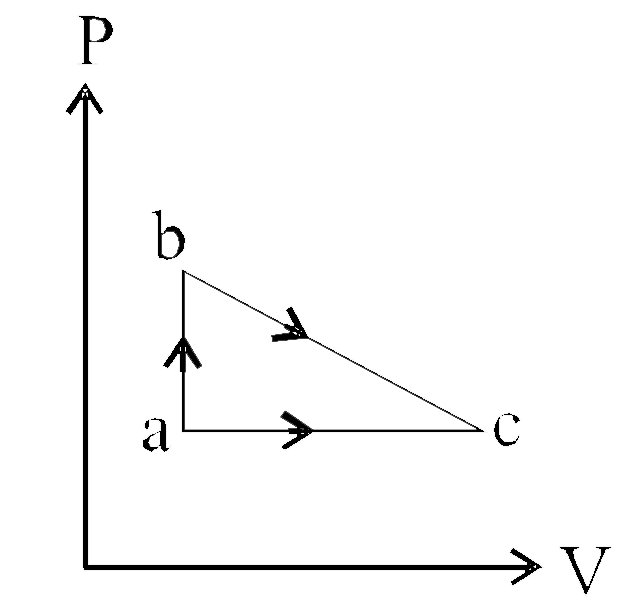
- A gas can be taken from state a to c by two different reversible proce...
Text Solution
|
- The rms speed of helium gas at 27^(@)C and 1 atm pressure is 900ms^(-1...
Text Solution
|
- A gas is enclosed in a cylindrical piston. When the gas is heated from...
Text Solution
|
- Two containers are filled with gases at the same temperature. In the c...
Text Solution
|
- Explain the temperature and pressure of a gas according to the kinetic...
Text Solution
|
- One mole of an ideal gas is taken through the cyclic process as shown ...
Text Solution
|
- Two moles of helium gas are taken over the cycle ABCDA, as shown in th...
Text Solution
|
- Five moles of helium are mixed with two moles of hydrogen to form a mi...
Text Solution
|
- Hydrogen gas is contained in a rigid container. A second rigid contain...
Text Solution
|
- Three processes compose a thermodynamic cycle shown in the accompanyin...
Text Solution
|
- A certain quantity of an ideal gas initially at temperature T(0).press...
Text Solution
|
- A certain quantity of an ideal gas initially at temperature T(0).press...
Text Solution
|
- A thermodynamic system is taken from an initial state X along the path...
Text Solution
|
- A thermodynamic system is taken from an initial state X along the path...
Text Solution
|
- The below P-V diagram represents the thermodynamic cycle for one mole ...
Text Solution
|
- A container with insulating walls is divided into two equal parts by a...
Text Solution
|
- If C(P) and C(V) denote the specific heats of nitrogen per unit gram a...
Text Solution
|
- A new monatomic ideal gas is discovered and named Wellsium. A pure 4-m...
Text Solution
|
- A sample of gas is caused to go through the cycle shown in the PV diag...
Text Solution
|
- 0.02 moles of an ideal gas undergo a cycle consisting of an isochoric ...
Text Solution
|