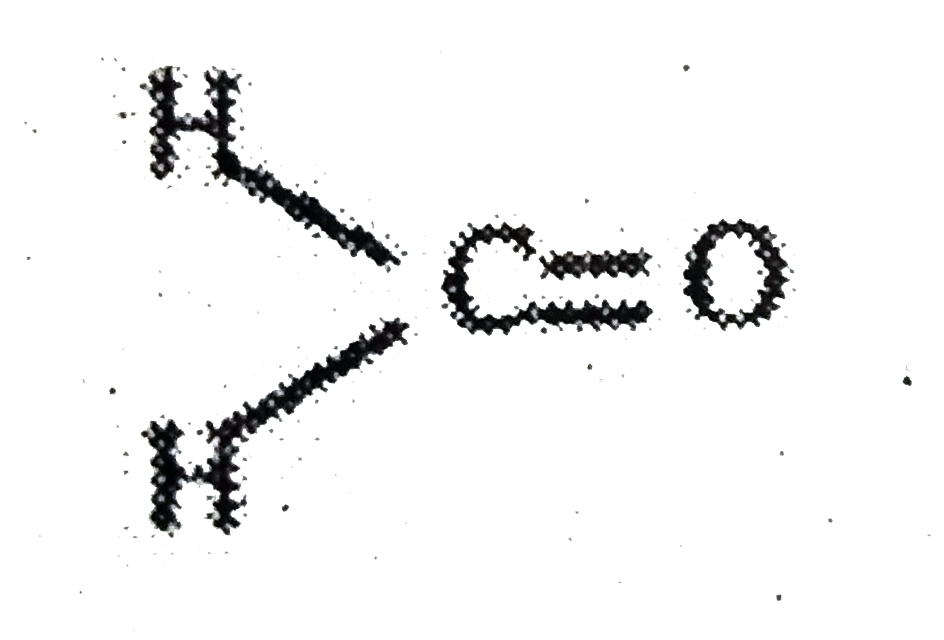
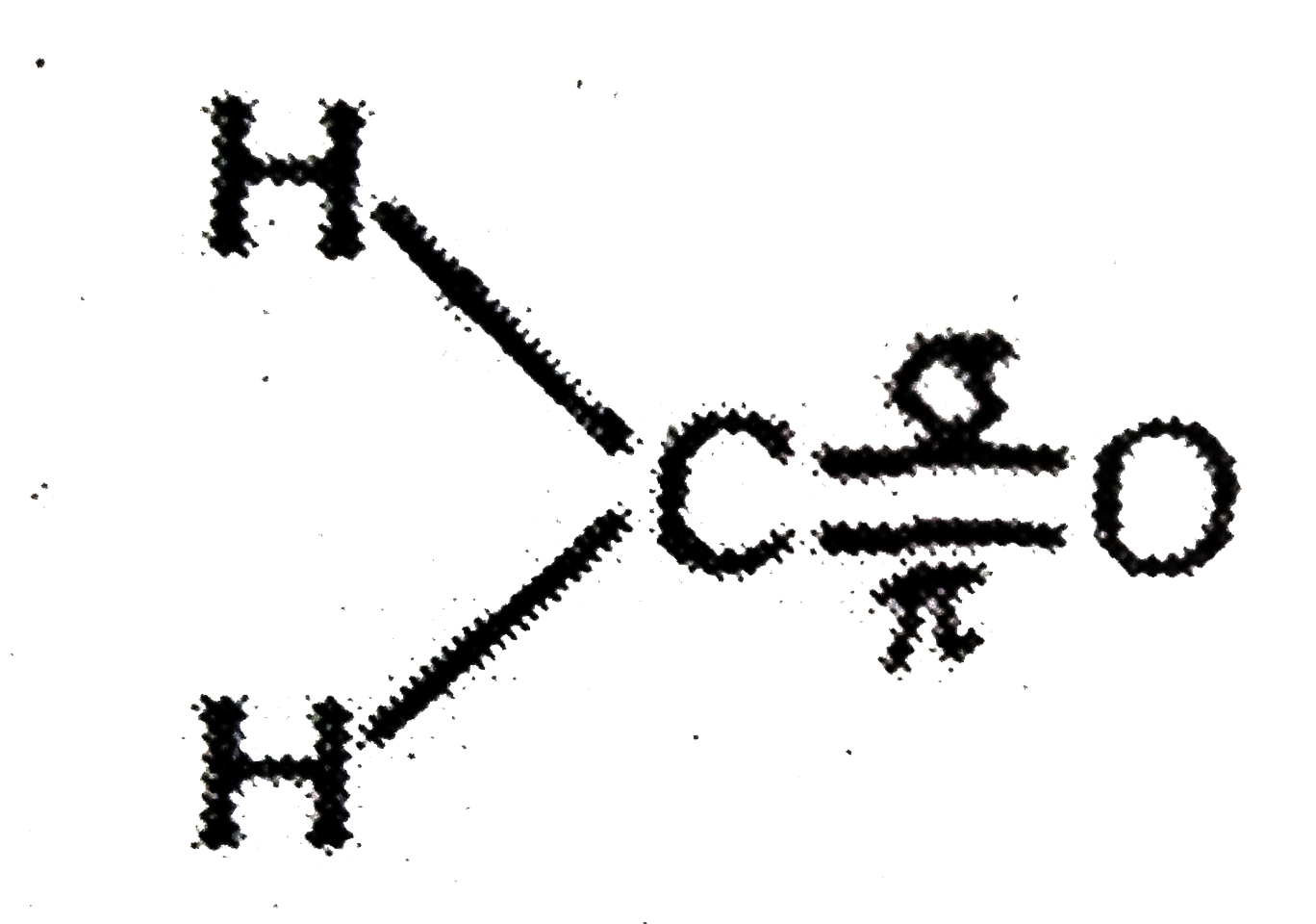
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Assertion:In formaldehyde, all the four atoms are in the same plane ...
Text Solution
|
- Assertion : All the carbon atoms of but-2-ene lie in one plane. Reason...
Text Solution
|
- Assertion : All the carbon atoms of but-2-ene lie in one plane. Reason...
Text Solution
|
- Assertion:In formaldehyde, all the four atoms are in the same plane ...
Text Solution
|
- एल्केनो में सभी कार्बन परमाणु sp^3 संकरित होते हैं।
Text Solution
|
- Assertion : In formaldehyde all the four atoms lie in one plane. Reaso...
Text Solution
|
- (A) All the hydrogen atoms in but-2-ene lie in one plane. (R) All the ...
Text Solution
|
- In an sp-hybridized carbon atom,
Text Solution
|
- Assertion : In methanal, all the four atoms are in the same plane. Rea...
Text Solution
|