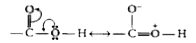Text Solution
Verified by Experts
Topper's Solved these Questions
CARBOXYLIC ACIDS
ARIHANT PUBLICATION|Exercise PART II QUESTIONS FOR PRACTICE (SHORT ANSWER TYPE II QUESTIONS)|1 VideosCARBOXYLIC ACIDS
ARIHANT PUBLICATION|Exercise PART II QUESTIONS FOR PRACTICE (LONG ANSWER TYPE QUESTION) |2 VideosCARBOXYLIC ACIDS
ARIHANT PUBLICATION|Exercise PART II QUESTIONS FOR PRACTICE (VERY SHORT ANSWER TYPE QUESTIONS)|8 VideosARYL DIAZONIUM SALTS
ARIHANT PUBLICATION|Exercise CHAPTER PRACTICE (LONG ANSWER TYPE QUESTIONS) 7 MARKS |2 VideosCHEMICAL KINETICS
ARIHANT PUBLICATION|Exercise CHAPTER PRACTICE (Long Answer Type Questions)|8 Videos
Similar Questions
Explore conceptually related problems
ARIHANT PUBLICATION-CARBOXYLIC ACIDS -PART II QUESTIONS FOR PRACTICE (SHORT ANSWER TYPE I QUESTIONS)
- How is benzoic acid converted to benzaldehyde?
Text Solution
|
- Arrange the following compounds in the increasing order of their prope...
Text Solution
|
- Arrange the following compounds in the increasing order of their prope...
Text Solution
|
- Carboxylic acids contain carbonyl group but do not show the nucleophil...
Text Solution
|
- Identify A, B, C and D. CH(3)COOHoverset(NH(3))rarrAoverset(Delta)ra...
Text Solution
|
- Compound A was prepared by the oxidation of compound B with alkaline K...
Text Solution
|
- Identify A, B, C and D C(6)H(5)COOHoverset(PCl(5))rarrAundersetunder...
Text Solution
|
- Give the simple chemical tests to distinguish between the following pa...
Text Solution
|
- Give the simple chemical tests to distinguish between the following pa...
Text Solution
|