Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ARIHANT PUBLICATION-SAMPLE PAPER 4 -GROUP C
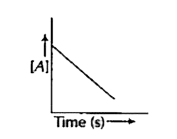
- Consider the reaction, A overset(k)to P. The change in the concentrat...
Text Solution
|
- The rate constant for a first order reaction is 60" s"^(-1). How much ...
Text Solution
|
- State the relationship amongst cell constant of a cell resistance of t...
Text Solution
|
- An aldehyde A(C(11)H(8)O) which does not undergo self aldol condensa...
Text Solution
|
- Haloarenes are much less reactive than haloalkanes towards nucleophili...
Text Solution
|
- Describe the mechanism of acid catalysed dehydration of ethanol to yie...
Text Solution
|
- Square planar complexes of MX(2)L(2) type with coordination number of ...
Text Solution
|