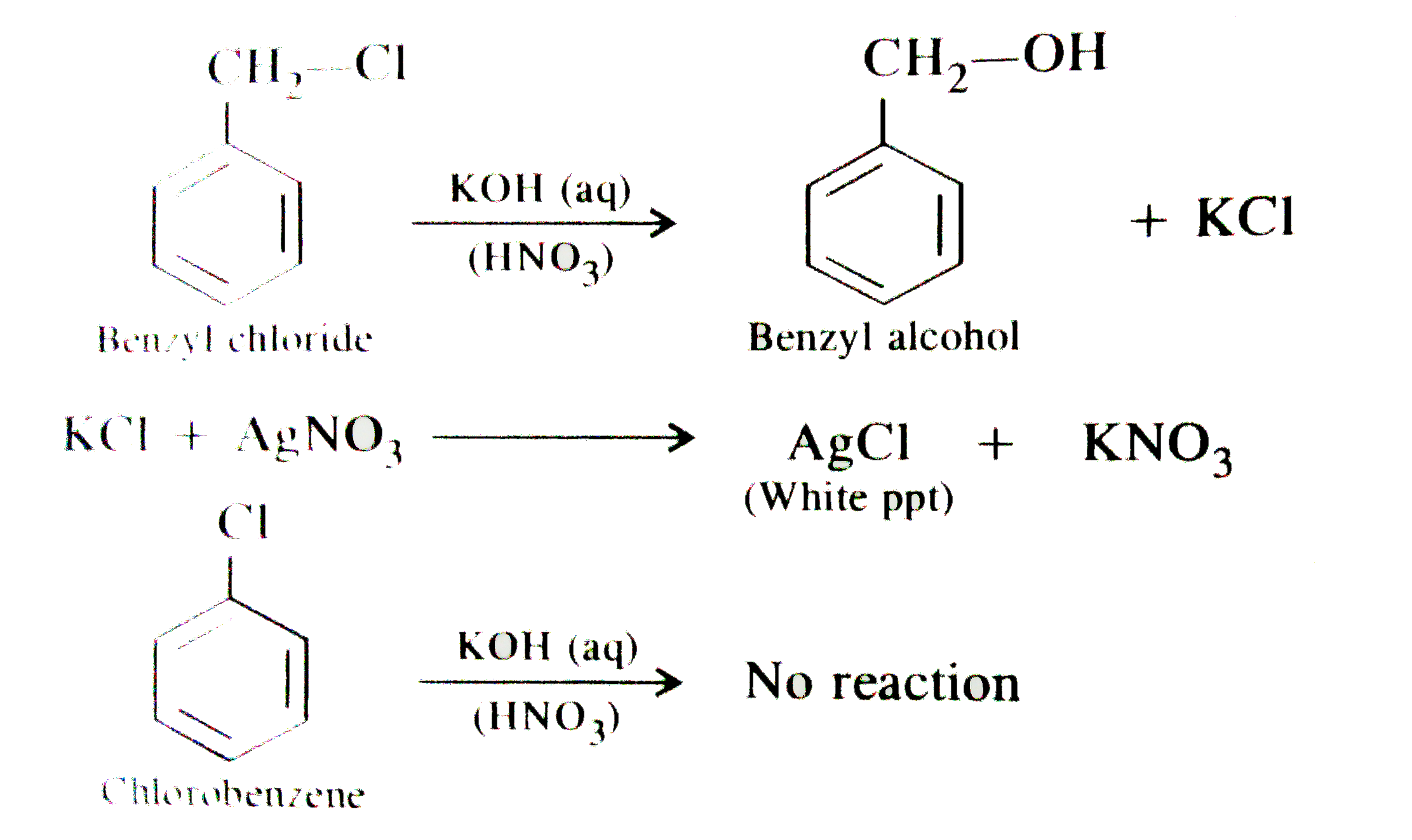Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- when benzyl chloride is boiled with aqueous KOH solution and the solut...
Text Solution
|
- A white precipitate insoluble in cone HNO(3) is formed when aqueous so...
Text Solution
|
- Aqueous solution of a salt when treated with AgNO(3) solution gives a ...
Text Solution
|
- when benzyl chloride is boiled with aqueous KOH solution and the solut...
Text Solution
|
- Out of benzyl chloride and chlorobenzene, which will give a white prec...
Text Solution
|
- When an aqueous solution of potassium chloride is added to an aqueous ...
Text Solution
|
- निम्नलिखित में से कौन जलीय विलयन में AgNO(3) के साथ श्वेत अवक्षेप देगा...
Text Solution
|
- Which one of the following will react with AgNO(3) solution to form a ...
Text Solution
|
- When an aqueous solution of potassium chloride is added to an aqueous ...
Text Solution
|