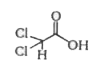
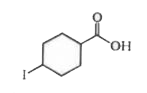
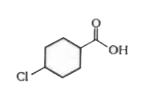
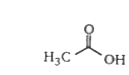A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Select the acid with the highest Ka (i.e., lowest pKa )
Text Solution
|
- Among hypohalous acid the acid with highest Ka value is
Text Solution
|
- Among the following acids, which has the lowest pKa value-
Text Solution
|
- Among the following acids, which has the lowest pKa value-
Text Solution
|
- নিম্নলিখিত কোনটির pKa - এর মান সর্বনিন্ম—
Text Solution
|
- Among the following which has lowest pKa value?
Text Solution
|
- Which of the following acids pKa The value is the lowest.
Text Solution
|
- Among the following acids which has the lowest pKa value
Text Solution
|
- Which acid has lowest value of pKa ?
Text Solution
|