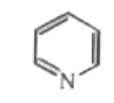
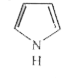
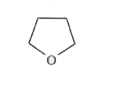
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Among the following compounds, the most basic compound is :
Text Solution
|
- Among the following the least basic compound is
Text Solution
|
- Among the following substituted pyridines, the most basic compound is-
Text Solution
|
- Among the following compounds, most basic is
Text Solution
|
- Among the following compounds most basic amino acid is :
Text Solution
|
- The most basic compound among the following is-
Text Solution
|
- The order of basicity among the following compounds is
Text Solution
|
- Of the following compounds, the most basic is
Text Solution
|
- The most basic compound in the following is
Text Solution
|