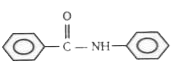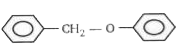To determine which compound allows for electrophilic aromatic substitution (EAS) to occur on the phenyl ring present on the left-hand side, we need to analyze the substituents attached to the aromatic rings in each option. Here’s a step-by-step solution:
### Step 1: Understand Electrophilic Aromatic Substitution (EAS)
Electrophilic aromatic substitution occurs when an electrophile attacks an aromatic ring. The reaction is favored by electron-rich aromatic systems, as they can stabilize the positive charge that develops during the reaction.
### Step 2: Identify Electron-Donating and Electron-Withdrawing Groups
- **Electron-Donating Groups (EDGs)**: These groups increase the electron density of the aromatic ring, making it more reactive towards electrophiles. Common EDGs include -OH, -OCH₃, -NH₂, etc.
- **Electron-Withdrawing Groups (EWGs)**: These groups decrease the electron density of the aromatic ring, making it less reactive towards electrophiles. Common EWGs include -NO₂, -CN, -COOH, etc.
### Step 3: Analyze Each Compound
Let’s consider the four options one by one:
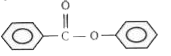
1. **Option 1**: Contains an electron-donating group on the left ring and an electron-withdrawing group on the right ring. The left ring becomes electron-rich, but the right ring is more electron-rich due to the EDG. EAS will occur on the right ring.
2. **Option 2**: Similar to option 1, the left ring has an EDG, but the right ring is more electron-rich. EAS will occur on the right ring.
3. **Option 3**: Again, the left ring has an EDG, while the right ring is more electron-rich. EAS will occur on the right ring.
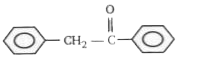
4. **Option 4**: The left ring has an electron-withdrawing group, making it electron-deficient, while the right ring has an EDG, making it electron-rich. In this case, the left ring is more electron-rich than the right ring due to the presence of the EDG on the left ring, allowing EAS to occur on the left ring.
### Step 4: Conclusion
Based on the analysis, the only compound where electrophilic aromatic substitution occurs on the left-hand side phenyl ring is **Option 4**.
### Final Answer
**Option 4** is the correct choice where electrophilic aromatic substitution takes place in the phenyl ring present on the left-hand side.
---