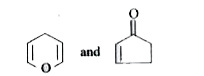
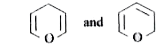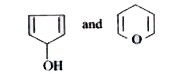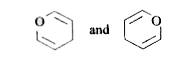A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following pairs of structures does not represent isomers?
Text Solution
|
- Which of the following pairs of structures represent facial and meridi...
Text Solution
|
- Which of the following pairs of strutures does not represent resonatin...
Text Solution
|
- Which of the following pairs of structures does not represent valid re...
Text Solution
|
- Which of the following pairs of structures represents facial and merid...
Text Solution
|
- Which of the following pairs represents constitutional isomers?
Text Solution
|
- Which of the following pairs represents constitutional isomers ?
Text Solution
|
- Which of the following pairs does not represent a pair of isomers?
Text Solution
|
- निम्न में कौनसा युग्म बन्धक समावयवी प्रदर्शित करता है
Text Solution
|