To determine which of the given compounds does not contain a plane of symmetry, we will analyze each option step-by-step.
### Step 1: Understand the Concept of Plane of Symmetry
A plane of symmetry is an imaginary plane that divides a molecule into two equal halves, such that each half is a mirror image of the other. If a compound has a plane of symmetry, it means that one side of the molecule can be reflected onto the other side.
**Hint:** Remember that for a molecule to have a plane of symmetry, both halves must be identical in shape and arrangement.
### Step 2: Analyze Each Compound
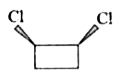
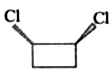
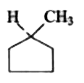
1. **Option 1:** Examine the first compound. If you visualize cutting through the molecule, you will find that both halves are mirror images of each other. Therefore, this compound has a plane of symmetry.
**Hint:** Look for identical functional groups or arrangements on either side of the cut.
2. **Option 2:** Now, consider the second compound. Similar to the first, if you cut through this compound, you will also find that both halves are mirror images. Thus, this compound also has a plane of symmetry.
**Hint:** Check if the arrangement of atoms is symmetrical across the proposed plane.
3. **Option 3:** For the third compound, when you attempt to cut through it, you will notice that the distances between certain atoms are not equal on both sides. This indicates that the halves are not mirror images of each other, meaning this compound does not have a plane of symmetry.
**Hint:** Focus on the distances and arrangements of atoms; if they differ, the compound likely lacks symmetry.
4. **Option 4:** Finally, analyze the fourth compound. If you cut through this compound, you will find that it does have a plane of symmetry, as the halves are mirror images.
**Hint:** Again, look for symmetry in the arrangement of atoms.
### Step 3: Conclusion
After analyzing all four options, we conclude that **Option 3** is the compound that does not contain a plane of symmetry.
**Final Answer:** Option 3 does not contain a plane of symmetry.