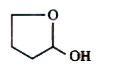
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following compounds give positive test with Tollen's reag...
Text Solution
|
- Which of the following acid give positive Tollen's reagent test.
Text Solution
|
- Which of the following compounds give positive Tollen's test ?
Text Solution
|
- Which of the following compounds will give positive Tollen's test ?
Text Solution
|
- OF the following compounds , how many would gives positive test with T...
Text Solution
|
- The pair of compounds in which both the compounds give positive test w...
Text Solution
|
- Which of the following compounds will NOT give the positive test for T...
Text Solution
|
- Which of the following compounds will give positive test with Tollen's...
Text Solution
|
- The pair of compounds in which both the compounds give positive test w...
Text Solution
|