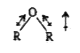Text Solution
Verified by Experts
Topper's Solved these Questions
ALCOHOLS , PHENOLS AND ETHERS
BETTER CHOICE PUBLICATION|Exercise 13 . 6 . 2 . PHYSICAL PROPERTIES |1 VideosALCOHOLS , PHENOLS AND ETHERS
BETTER CHOICE PUBLICATION|Exercise 14 . 6 . 2 . PHYSICAL PROPERTIES |1 VideosALCOHOLS , PHENOLS AND ETHERS
BETTER CHOICE PUBLICATION|Exercise 11 . 6 . 2 . PHYSICAL PROPERTIES |1 VideosALTERNATING CURRENTS
BETTER CHOICE PUBLICATION|Exercise MOST EXPECTED QUESTIONS|11 Videos
Similar Questions
Explore conceptually related problems