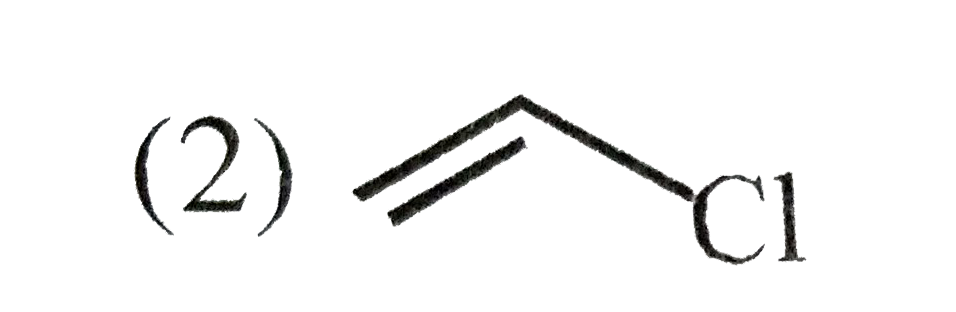
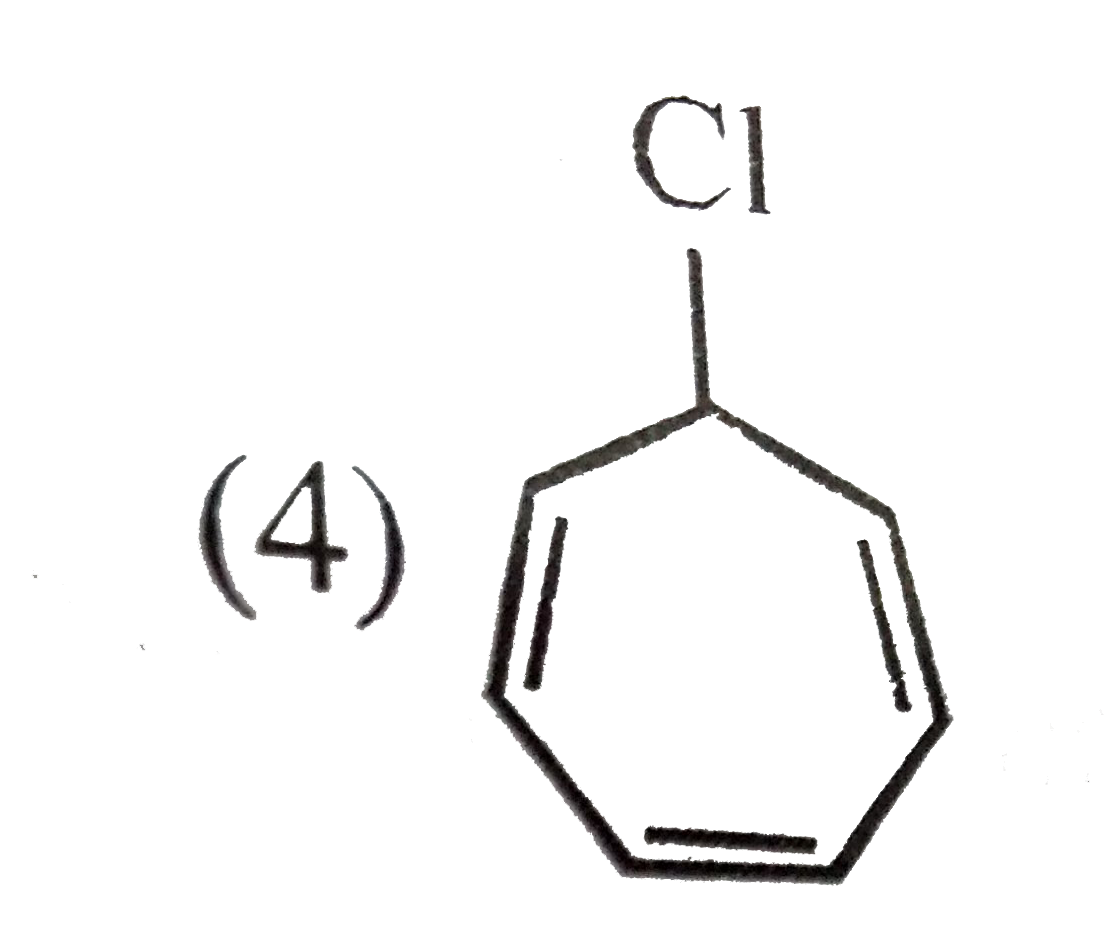
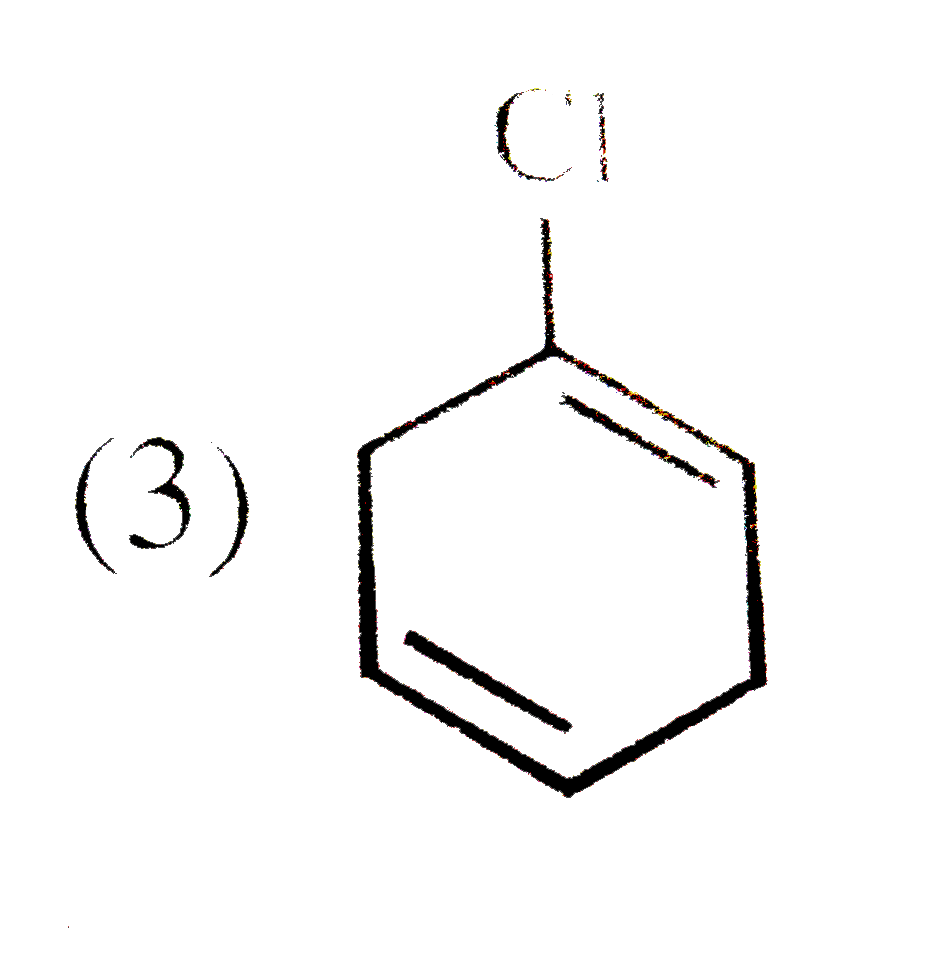A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-CHEMISTRY AT A GLANCE-ORGANIC CHEMISTRY
- Which of the following compound undergo hydrolysis most easily :
Text Solution
|
- Consider the reaction CH(3) CH(2) CH(2) Br + NaCN rarr CH(3) CH(2) C...
Text Solution
|
- Which of the following compound will give curdy precipiate with AgNO(3...
Text Solution
|
- Arrange the following group in order of their decreasing leaving abili...
Text Solution
|
- (CH(3))(2)"CHCl + Nal" rarr (CH(3))(2)"CHl+ NaCl" The above reaction...
Text Solution
|
- The correct order for E(2) reaction with alc. KOH will be :-
Text Solution
|
- Which of the following reaction is correct :
Text Solution
|
- Which one of the following compounds is more reactive towards S(N)2 re...
Text Solution
|
- Which of the following statement is incorrect :-
Text Solution
|
- Which of the following is correctly match :- (A) Chloramphenicol to ...
Text Solution
|
- C(2)F(3)Cl(3) is named as :-
Text Solution
|
- Which one of the following will give racemised product in C(2)H(5)OH...
Text Solution
|
- Arrange the following group in order of their nucleophilic strength :-
Text Solution
|
- Which of the following molecule would have a carbon halogen bond most ...
Text Solution
|
- Which of the following is the most reactive towards nucleophilic subst...
Text Solution
|
- An S(N)2 reaction at an asymmetric carbon of a compound always g...
Text Solution
|
- The synthesis of alkyl fluorides is best accomplished by :
Text Solution
|
- The reagent that brings about the conversion of butanol to chlorobutan...
Text Solution
|
- Which of the following is correct order of order of dipole moment :-
Text Solution
|
- DDT is manufactured by acid catalysed condensation of :
Text Solution
|