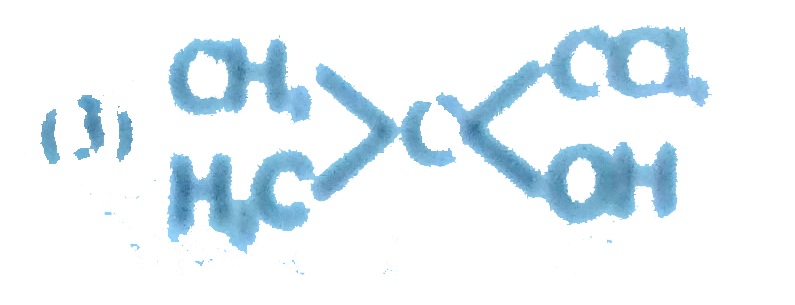A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-CHEMISTRY AT A GLANCE-ORGANIC CHEMISTRY
- ?underset(2.H(2)O)overset(1.Delta//DIBAL-H)(to)CH(3)-CH=CH-CH(2)-CH(2)...
Text Solution
|
- CH(3)-underset(O)underset(||)(C)-CH(3)+underset(("semicarbazide"))(NH(...
Text Solution
|
- Ph-underset(O)underset(||)(C)-CH(3)overset(NaOCl)(to)A+ "salt" A+CH(...
Text Solution
|
- Complete the following reaction
Text Solution
|
- CH(3)-CH=CH-underset(O)underset(||)(C)-CH(3)+NH(2)-OHoverset(H^(o+))(t...
Text Solution
|
- Give eletrophilicity order of following carbonyl compounds (I) Benz...
Text Solution
|
- Complete the following reaction
Text Solution
|
- Complete the following reaction
Text Solution
|
- CH(3)CH(2)COOHCl(2)overset("RedP")(to)Aunderset(Delta)overset(NaOH//Ca...
Text Solution
|
- Assertion:- The carbonyl carbon is a electrophilic and carbonyl oxygen...
Text Solution
|
- Hydrolysis of which of the following gives acid at fastest rate :
Text Solution
|
- Complete the following reaction
Text Solution
|
- R is-
Text Solution
|
- H(3)C-COOHunderset(Delta)overset(P(2)O(5))(to)Aoverset(PCl(5))(to)Bund...
Text Solution
|
- Which of the following is not correctly matched
Text Solution
|
- Identify the end product : CH(3)CH(2)CH(2)CHOoverset(Ammonical AgNO(...
Text Solution
|
- Complete the following reaction
Text Solution
|
- What is Sandmeyer reaction ? Illustrate with a suitable example.
Text Solution
|
- Arrange the following compounds in increasing order of their boiling p...
Text Solution
|
- An amine reacts with C(6)H(5)SO(2)Cl and the product is soluble in alk...
Text Solution
|