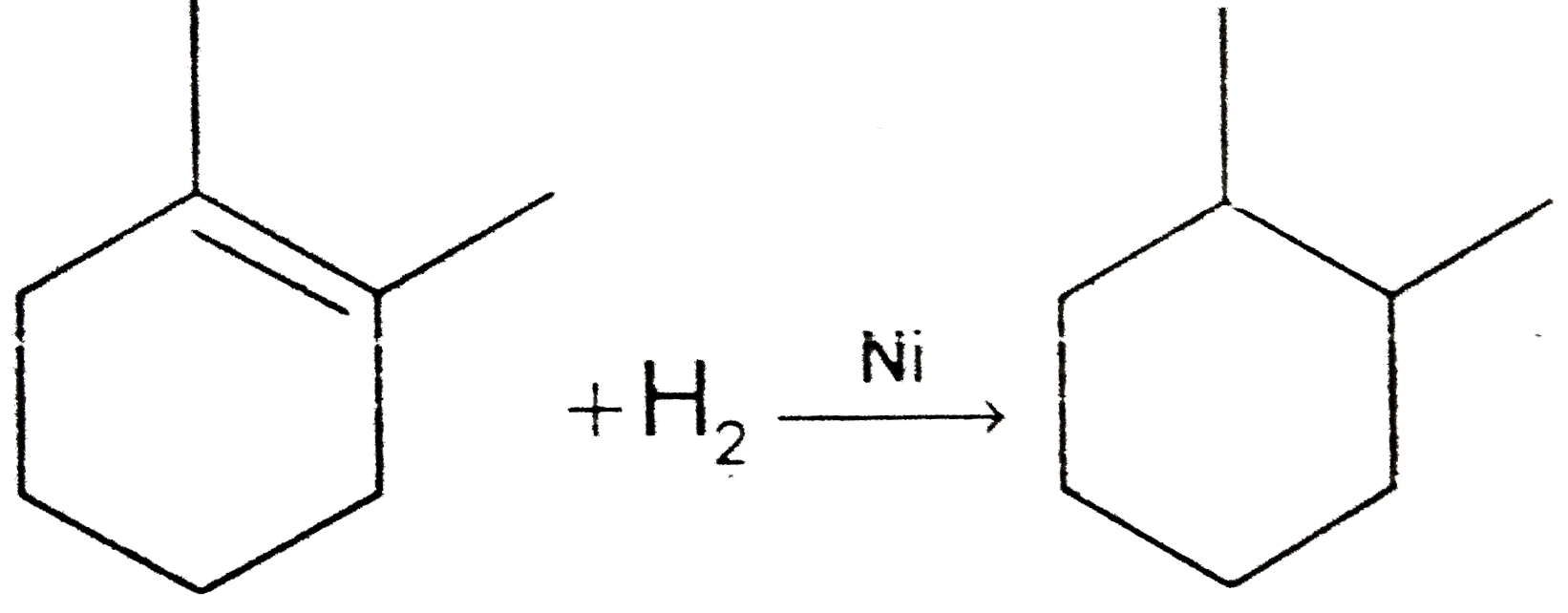
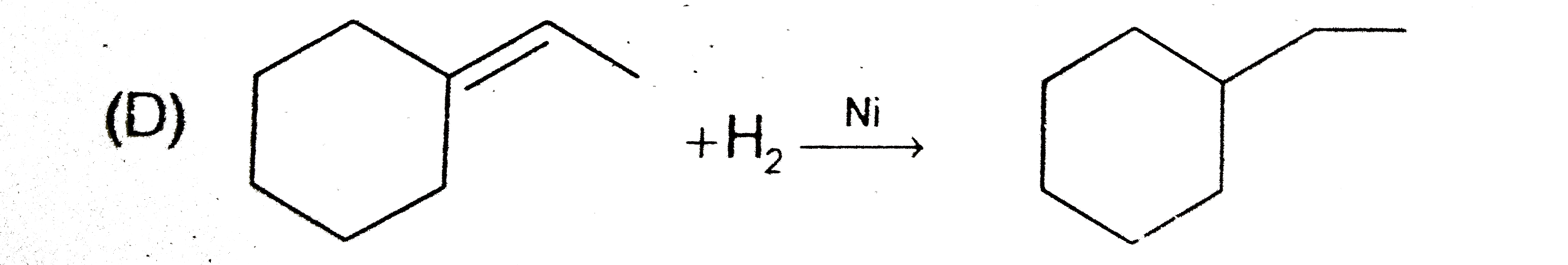A
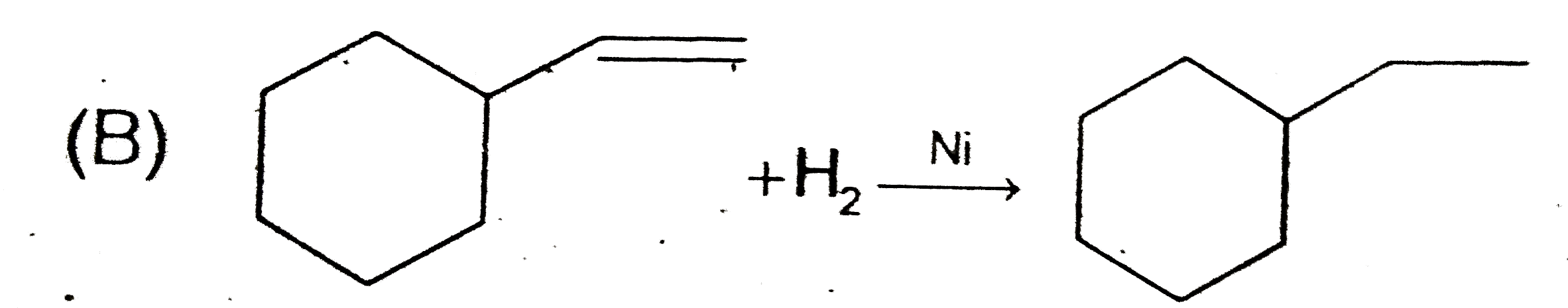
B
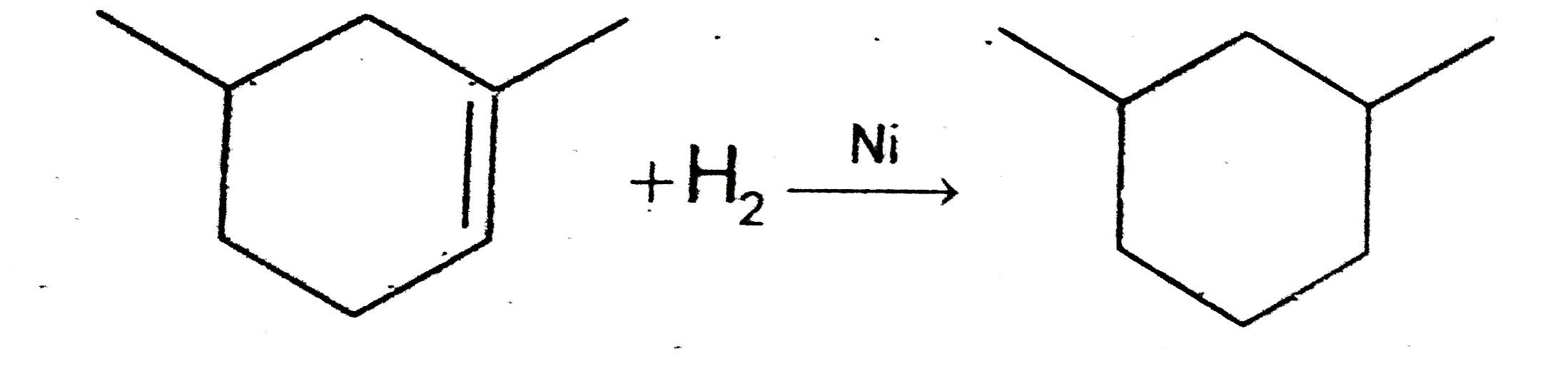
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following reaction liberates maximum amount of heat energ...
Text Solution
|
- Which of the following transitions involves maximum amount of energy?
Text Solution
|
- Maximum amount of energy/ATP is liberated on oxidation of
Text Solution
|
- In which of the following reactions, the heat liberated is known as ''...
Text Solution
|
- The chemical reaction in which heat is liberated are called ……….. rea...
Text Solution
|
- निम्नलिखित में से कौनसी इकाई ऊर्जा की अधिकतम मात्रा को निरूपित करती है
Text Solution
|
- Which of the following reaction involves the liberation of energy?
Text Solution
|
- In which of the following combinatins of HCL and NaOH, the heat energy...
Text Solution
|
- Which of the following reaction involves the liberation of energy?
Text Solution
|