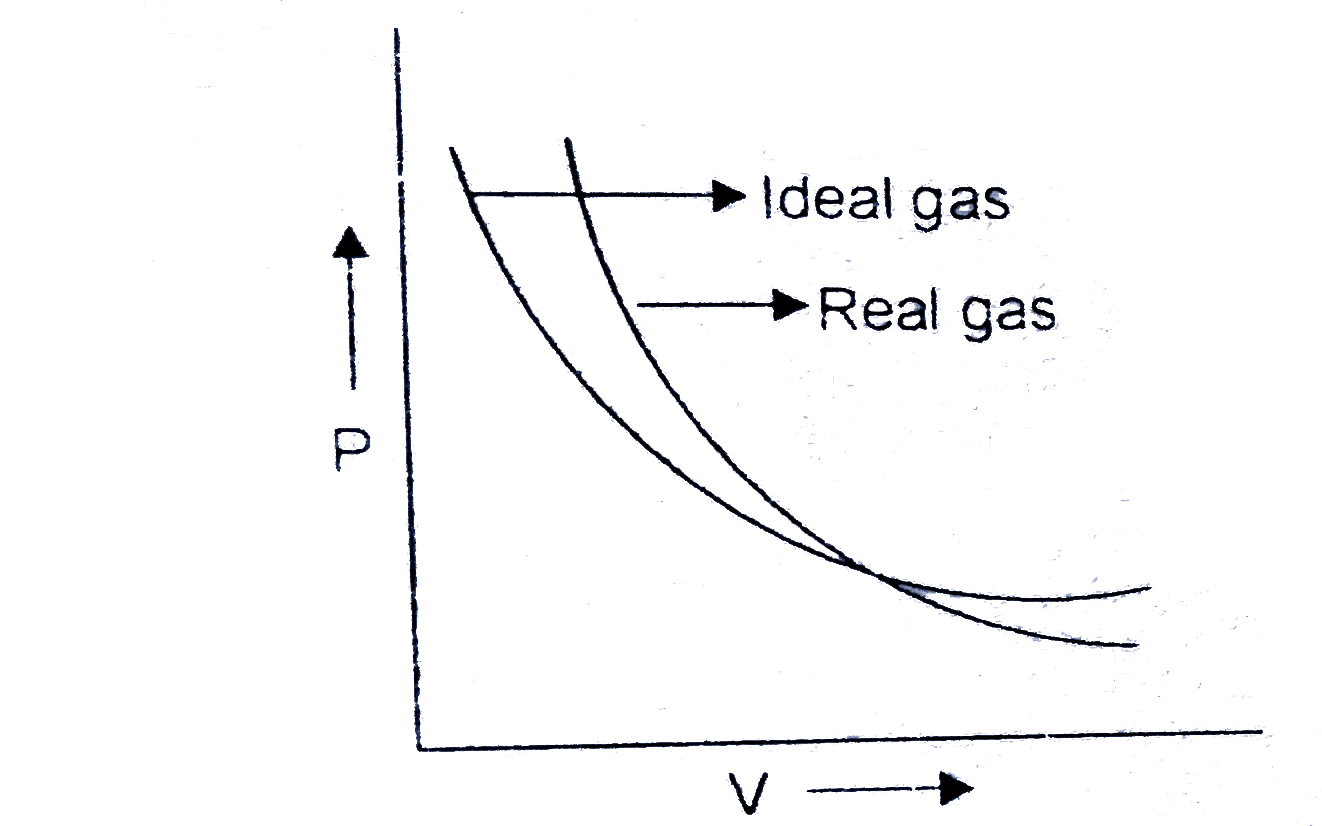
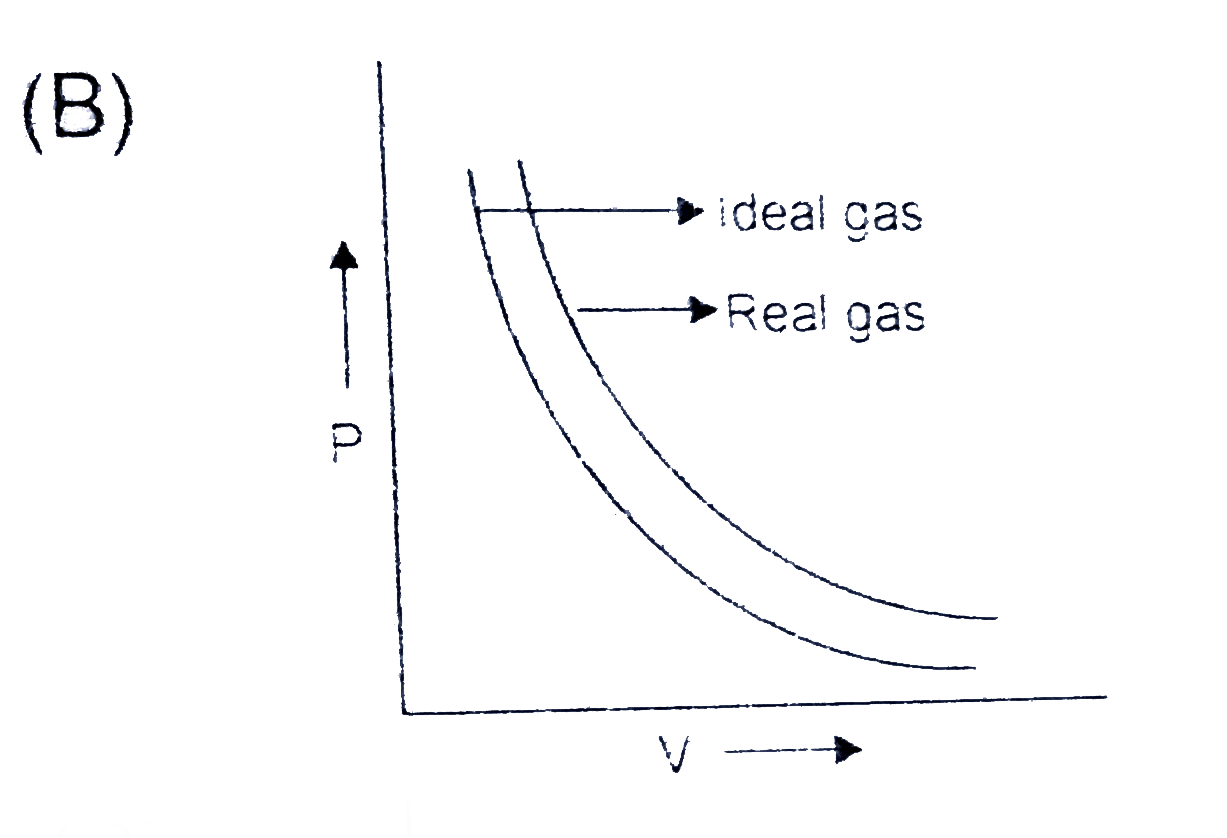
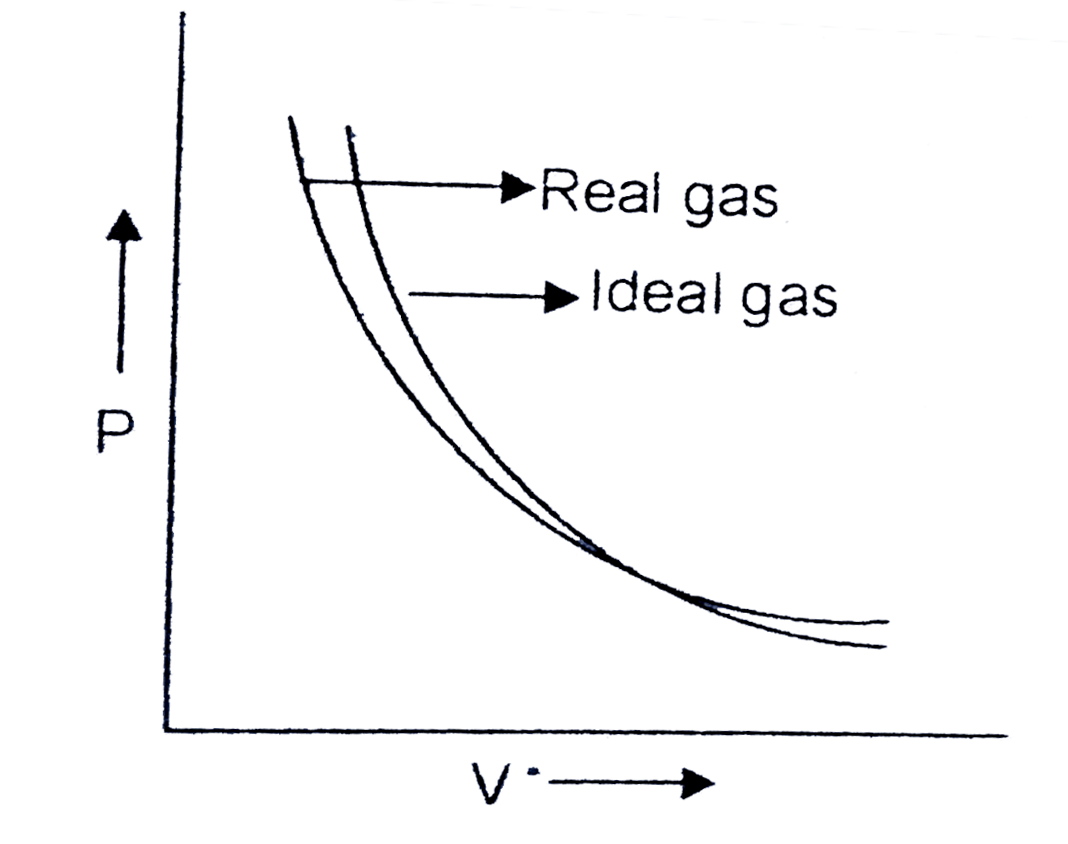
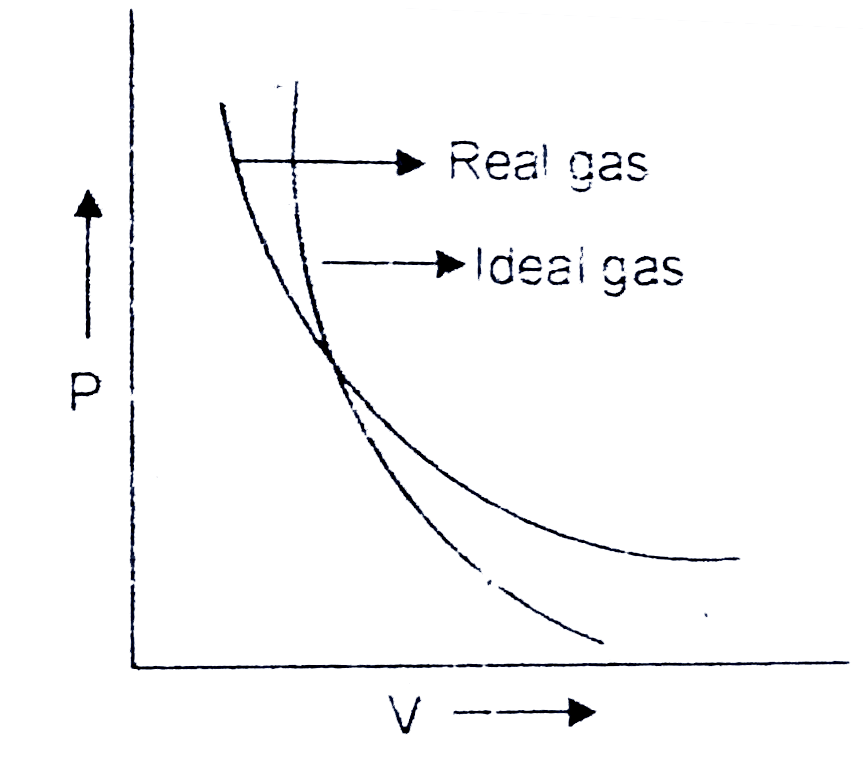A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following graph is correct for real gases other than hydr...
Text Solution
|
- Which of the following statements is/are correct about real gases?
Text Solution
|
- Which of the following are correct for real gases?
Text Solution
|
- Which of the following graphs regarding Maxwell distribution of speed ...
Text Solution
|
- Which of the following gases will diffuse fastest ? Nitrogen, Oxygen...
Text Solution
|
- Which is/are correct for real gases?
Text Solution
|
- निम्नलिखित प्रश्नो में सही विकल्प का चयन कीजिए - हाइड्रोजन अणु से ...
Text Solution
|
- Which of the following is(are) correct for real gases?
Text Solution
|
- Two balloons of the same volume are filled with two gases at the same ...
Text Solution
|