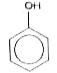
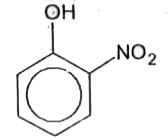
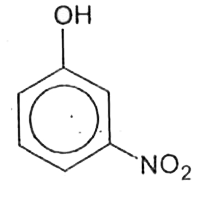
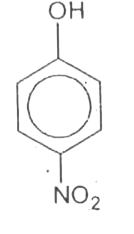
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The acidic character of alcohols is due the polar nature of O-H bond. ...
Text Solution
|
- Phenols and alcohols have both the same functional group (-OH) but phe...
Text Solution
|
- Phenols and alcohols have both the same functional group (-OH) but phe...
Text Solution
|
- Phenols and alcohols have both the same functional group (-OH) but phe...
Text Solution
|
- Assertion : Solubility of alcohols in water decreases with the increas...
Text Solution
|
- (A ) Acidic character of alcohols follows the order : Primary gt Sec...
Text Solution
|
- The acidic character of alcohols is due the polar nature of O-H bond. ...
Text Solution
|
- The acidic character of alcohols is due the polar nature of O-H bond. ...
Text Solution
|
- Assertion(A): Primary alcohols are more acidic than tertiary alcohol. ...
Text Solution
|