Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
OMEGA PUBLICATION-GENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS -MULTIPLE CHOICE QUESTIONS
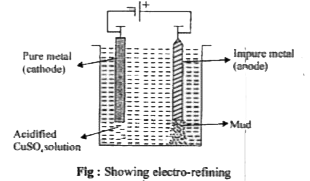
- How is copper purified by electro - refining ?
Text Solution
|
- An ore of aluminium is
Text Solution
|
- Which of the following is used as a depressant in froth floatation pro...
Text Solution
|
- The reducing agent used in thermite process is
Text Solution
|
- Cupellation process is used in the metallurgy of
Text Solution
|
- Which of the following is not an ore ?
Text Solution
|
- Electrolytic reduction method is used in the ex- traction of
Text Solution
|
- The main fuction of roasting is
Text Solution
|
- In blast furnace, iron oxide is reduced by
Text Solution
|
- Cassiterite is an ore of
Text Solution
|
- The process of converting hydrated alumina into anhydrous alumina is c...
Text Solution
|
- The chemical formula of copper pyrite is: CuFeS2, Cu2S, Cu2O, CuCO3.Cu...
Text Solution
|
- Malachite is an ore of,
Text Solution
|