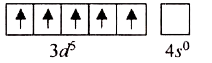Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
OMEGA PUBLICATION-THE D- AND F-BLOCK ELEMENTS-MULTIPLE CHOICE QUESTIONS
- Why does Mn(II) shows maximum paramagnetic character among the dival...
Text Solution
|
- Which element belongs to d-block elements ?
Text Solution
|
- The transition elements have a general electronic configuration of
Text Solution
|
- The correct ground state electronic configuration of chromium atom (Z...
Text Solution
|
- Which of the following electronic configuration is that of a transiti...
Text Solution
|
- Which of the following elements is alloyed with copper to form brass?
Text Solution
|
- Copper can be extracted from
Text Solution
|
- Argentite is an ore of
Text Solution
|
- The process used for the extraction of gold is
Text Solution
|
- Important ore of zinc is
Text Solution
|
- The inner transition elements are the elements in which the added elec...
Text Solution
|
- Transition elements
Text Solution
|
- The tendency of the transition elements to form coloured compounds is...
Text Solution
|
- Which of the following is diamagnetic ?
Text Solution
|
- The common oxidation state of the elements of lanthanide series is
Text Solution
|
- Which of the following is a lanthanide ?
Text Solution
|
- For the process Cu(g) to Cu^+ (g) + e^- , the electron is to be...
Text Solution
|
- Paramagnetism is a property of
Text Solution
|
- The first ionisation energy of silicon is lower than that of
Text Solution
|
- Which of the following electronic configurations will have the lowest...
Text Solution
|
- In general, the melting and boiling point of transition metals
Text Solution
|