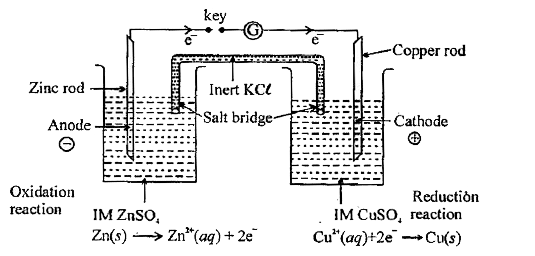
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
OMEGA PUBLICATION-REDOX REACTIONS -MULTIPLE CHOICE QUESTIONS (MCQs)
- Write four differences between galvanic (or electrochemical) cell and ...
Text Solution
|
- Electrolysis involves oxidation and reduction respectively at
Text Solution
|
- The cathode in a galvanic cell and electrolytic cell is
Text Solution
|
- In a galvanic cell,
Text Solution
|
- K, Ca and Li metals may be arranged in the decreasing order of their s...
Text Solution
|
- The standard reduction potential of three metallic cations X, Y and Z ...
Text Solution
|
- Arrange the following in the decreasing order of their acidic strength...
Text Solution
|
- Calculate the oxidation number of P in phosphate ion.
Text Solution
|
- Calculate the oxidation number of P in H3PO3
Text Solution
|
- Calculate the oxidation number of C in C2H6
Text Solution
|
- How many orbitals are present in f subshell.
Text Solution
|
- Galvanised iron sheets are coated with: C, Cu, Zn, Ni.
Text Solution
|
- According to classical concept, oxidation is a process of
Text Solution
|
- According to electronic concept, oxidation involves
Text Solution
|
- Write down the values of azimuthal quantum number possible for electro...
Text Solution
|
- How many orbitals are present in p subshell .
Text Solution
|
- How many orbitals are present in s subshell.
Text Solution
|
- In case of oxidation, there is
Text Solution
|
- Calculate mass of 2.7moles of C(12)H(22)O(11) (molar mass = 342 g/ mol...
Text Solution
|
- The most common oxidation state of an element is -2. The number of ele...
Text Solution
|
- In the conversion of K(2)Cr(2)O(7) "to K"(2)CrO(4), the oxidation numb...
Text Solution
|