Text Solution
Verified by Experts
Topper's Solved these Questions
HALOALKANES AND HALOARENES
ACCURATE PUBLICATION|Exercise 4 MARKS QUESTIONS|19 VideosHALOALKANES AND HALOARENES
ACCURATE PUBLICATION|Exercise TRUE AND FALSE (1 MARK)|10 VideosHALOALKANES AND HALOARENES
ACCURATE PUBLICATION|Exercise 1 MARK QUESTIONS|26 VideosELECTRO CHEMISTRY
ACCURATE PUBLICATION|Exercise Numbericals Practice|44 VideosIMPORTANT NAMING REACTION
ACCURATE PUBLICATION|Exercise DISTINGUISH TEST|5 Videos
Similar Questions
Explore conceptually related problems
ACCURATE PUBLICATION-HALOALKANES AND HALOARENES -2 OR 5 MARKS QUESTIONS
- Why a small amount of ethyl alcohol is usually added to chloroform bot...
Text Solution
|
- Why is Vinyl chloride less reactive than ethyl chloride ?
Text Solution
|
- Why is sulphuric acid not used during the reaction of alcohols with KI...
Text Solution
|
- Haloarenes are insoluble in water but soluble in benzene. Explain.
Text Solution
|
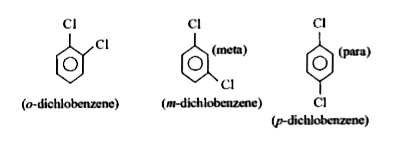
- The p-isomer of dichlorobenzene has higher melting point than O-and M-...
Text Solution
|
- What are ambident uncleophiles ? Explain with an example.
Text Solution
|
- Write short notes on Swarts reaction
Text Solution
|
- How Sandmeyer's reaction differs from Gattermann's reaction.
Text Solution
|
- Alkyl halides though polar, are immiscible with water, why ?
Text Solution
|
- Give different Enantiomers of Butanol-2.
Text Solution
|
- Give different Enantiomers of 2 chlorobutane.
Text Solution
|
- What is iodoform test ?
Text Solution
|
- How is DDT prepared from chlorobenzene ? Give the chemical equation on...
Text Solution
|
- Give the reaction chloroform with alcoholic KOH.
Text Solution
|
- Why is Wurtz reaction not suitable for the preparation of odd number a...
Text Solution
|
- Write two environmental effects of dichloromethane.
Text Solution
|
- Explain why Grignard reagents should be prepared under anhydrous con...
Text Solution
|
- Iodoform gives a precipitate with silver nitrate on heating while chlo...
Text Solution
|
- How will you convert. Anline into chlorobenzene.
Text Solution
|
- Complete the following reaction : CH3 CH2 Br + Ag CN to
Text Solution
|