Text Solution
Verified by Experts
Topper's Solved these Questions
ORGANIC COMPOUNDS CONTAINING NITROGEN COMPOUNDS
ACCURATE PUBLICATION|Exercise 1 MARK QUESTIONS|22 VideosMODEL TEST PAPER-9
ACCURATE PUBLICATION|Exercise SECTION-D (LONG ANSWER QUESTIONS) (TYPE II)|13 VideosP-BLOCK ELEMENTS
ACCURATE PUBLICATION|Exercise P-BLOCK ELEMENTS GROUP-18 (NOBLE GASES )(2 OR 5 MARK QUESTIONS)|17 Videos
Similar Questions
Explore conceptually related problems
ACCURATE PUBLICATION-ORGANIC COMPOUNDS CONTAINING NITROGEN COMPOUNDS-2 OR 4 MARKS QUESTIONS
- Write short note on Gabriel phthalimide synthesis. Why is it regarded ...
Text Solution
|
- Why do primary amines have higher boiling point than tertiary amines?
Text Solution
|
- Out of ammonia (NH(3)) and C(2)H(5)NH(2) which is more basic and why?
Text Solution
|
- Write short note on carbylamine reaction.
Text Solution
|
- Write Hinsberg's test to distinguish between 1^(@), 2^(@) and 3^(@) am...
Text Solution
|
- Why methyl amine has lower boiling point than methanol.
Text Solution
|
- Explain why secondary amines are more basic than primary amines.
Text Solution
|
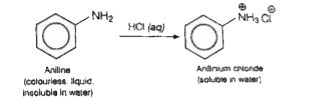
- Aniline dissolve in aqueous HCl. Why?
Text Solution
|
- How will you convert Aniline to benzene-diazonium chloride ?
Text Solution
|
- How will you convert benzene diazonium chloride to bromobenzene?
Text Solution
|
- Fill in the blanks- is used to separate two immiscible liquids.
Text Solution
|
- How will you convert aniline to benzene ?
Text Solution
|
- How will you convert benzene diazonium chloride to cynobenzene benzoni...
Text Solution
|
- Write short note on diazotisation reaction.
Text Solution
|
- How benzene diazonium chloride reacts with HNO(2) in the presence of c...
Text Solution
|
- Write the reaction of benzenediazonium chloride with Kl.
Text Solution
|
- How does benzene diazonium chloride react with : Phenol
Text Solution
|
- Write the reactions of benzenediazonium chloride with : Aniline
Text Solution
|
- How benzene diazonium chloride reacts with Cu Powder/HCl
Text Solution
|
- Write the reaction of benzene diazonium chloride with : CuCN
Text Solution
|