Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ACCURATE PUBLICATION-BOARD PAPER MARCH - 2020-SECTION - D
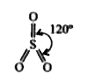
- ICI7 does not exist while IF7 exist. Why ?
Text Solution
|
- Give Neil Bartlett's experiment on reactivity of Xenon.
Text Solution
|
- SO3 has zero dipole moment. Why ?
Text Solution
|
- Why noble gases have zero electron affinity values ?
Text Solution
|
- What is formula of oleum ?
Text Solution
|
- Describe the preparation of potassium dichromate from iron chromite or...
Text Solution
|
- Why transition metals show catalytic properties?
Text Solution
|
- Give preparation of XeF6 and XeOF2.
Text Solution
|
- Explain why transition elements have high melting and boiling points ?
Text Solution
|
- What are the main consequences of lanthanoid contraction ?
Text Solution
|
- Write the following reactions Sandmeyer reaction
Text Solution
|
- Write the following reactions : Friedel Craft alkylation.
Text Solution
|
- Give the following reactions: Fitting reaction
Text Solution
|
- Difference between SN1 and SN2 reaction ?
Text Solution
|
- Define specific rotation ?
Text Solution
|
- Define: Racemic Mixture.
Text Solution
|
- Define Optical activity.
Text Solution
|
- Give two uses of chloroform.
Text Solution
|