Text Solution
Verified by Experts
Topper's Solved these Questions
SOLVED MODEL TEST PAPER-1
ACCURATE PUBLICATION|Exercise SECTION C|11 VideosSOLVED MODEL TEST PAPER-1
ACCURATE PUBLICATION|Exercise SECTION D|14 VideosSOLVED MODEL TEST PAPER-1
ACCURATE PUBLICATION|Exercise SECTION A(TRUE/FALSE BASED QUESTIONS)|5 VideosSOLVED SAMPLE PAPER, MARCH - 2021
ACCURATE PUBLICATION|Exercise SECTION -D |16 VideosSOLVED MODEL TEST PAPER-2
ACCURATE PUBLICATION|Exercise SECTION D|11 Videos
Similar Questions
Explore conceptually related problems
ACCURATE PUBLICATION-SOLVED MODEL TEST PAPER-1-SECTION B
- Most of transition metals show variable oxidation states. Explain
Text Solution
|
- Define ionisation isomerism. Give example. How can you distinguish bet...
Text Solution
|
- Briefly explain Linkage Groups.
Text Solution
|
- Write IUPAC name of K3 [Fe(CN)6].
Text Solution
|
- Give IUPAC names [Co(en)2Cl(NO2)]^+
Text Solution
|
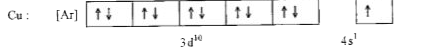
- Why is Copper considered as transition metal ?
Text Solution
|
- Write the difference between molecularity and order of reaction?
Text Solution
|
- The rate constant for a first order reaction Is 90 s^-1 .How much time...
Text Solution
|
- The rate constant for a first order reaction is 60 s^(-1). How much ti...
Text Solution
|
- What is the difference between e.m.f. and potential diffrence?
Text Solution
|
- H2S is a gas while H2O is liquid at room temperature·? Why ?
Text Solution
|
- Why SF6 is known but SH6 is not known ?
Text Solution
|
- Draw the structure of XeO3. Write its state of hybridisation ?
Text Solution
|
- 18 g of glucose is dissolved in 1 kg of water. At what temperature wil...
Text Solution
|
- How many grams of ethylene glycol (molar mass = 62) should be added to...
Text Solution
|
- State and explain Hess's law.
Text Solution
|
- Vapour pressure of liquid depends on which factors ?
Text Solution
|