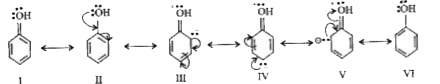
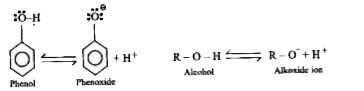Text Solution
Verified by Experts
Topper's Solved these Questions
SOLVED MODEL TEST PAPER-1
ACCURATE PUBLICATION|Exercise SECTION D|14 VideosSOLVED MODEL TEST PAPER-1
ACCURATE PUBLICATION|Exercise SECTION B|17 VideosSOLVED SAMPLE PAPER, MARCH - 2021
ACCURATE PUBLICATION|Exercise SECTION -D |16 VideosSOLVED MODEL TEST PAPER-2
ACCURATE PUBLICATION|Exercise SECTION D|11 Videos
Similar Questions
Explore conceptually related problems
ACCURATE PUBLICATION-SOLVED MODEL TEST PAPER-1-SECTION C
- The molar conductivities at infinite dilution for sodium acetate, hydr...
Text Solution
|
- Explain the following : Iodine is more soluble in KI solution than i...
Text Solution
|
- What are pseudohalogens ? Give example.
Text Solution
|
- Explain Reimer Tiemann reaction with one example.
Text Solution
|
- How will you convert chlorobenzene into phenol ?
Text Solution
|
- Why Phenols are more acidic than Alcohol ?
Text Solution
|
- Why solubility of alcohols in water decreases with increase in molecul...
Text Solution
|
- Why primary alcohols are more acidic than secondary alcohols?
Text Solution
|
- Ethers possess a dipole moment even if the alkyl groups in the molecul...
Text Solution
|
- A first order reaction takes 23.1 minutes for 50% conmpletion. Calcul...
Text Solution
|
- Rate constant for a first order reaction is 60 s ^(-1) . How much tim...
Text Solution
|