Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ACCURATE PUBLICATION-SOLVED MODEL TEST PAPER-1-SECTION D
- Write the following reaction: Wurtz Fittig Reaction
Text Solution
|
- Explain the following reactions: Balz Schiemann reaction.
Text Solution
|
- Write the following reactions : Friedel Craft alkylation.
Text Solution
|
- Why solubility of Haloalkanes in water is very low ?
Text Solution
|
- Give one use of freon.
Text Solution
|
- Why are haloarenes more stable than haloalkanes ?
Text Solution
|
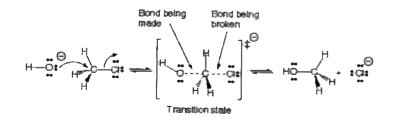
- Give the mechanism of substitution nucleophilic bimolecular, SN^2 reac...
Text Solution
|
- Define Optical activity.
Text Solution
|
- Transition metals have high melting and boiling points. Why ?
Text Solution
|
- How many unpaired electrons are present in Fe^+3 and Zn^+2.
Text Solution
|
- Why is La(OH)3 more basic than Lu(OH)3 ?
Text Solution
|
- Why are Mn^(2+) compounds more stable than Fe^(2+) compounds towards o...
Text Solution
|
- What is Lanthanide contraction ? What is the cause and consequences of...
Text Solution
|
- Why transition metals show catalytic properties?
Text Solution
|