Text Solution
Verified by Experts
Topper's Solved these Questions
SOLVED MODEL TEST PAPER-2
ACCURATE PUBLICATION|Exercise SECTION C|6 VideosSOLVED MODEL TEST PAPER-2
ACCURATE PUBLICATION|Exercise SECTION D|11 VideosSOLVED MODEL TEST PAPER-2
ACCURATE PUBLICATION|Exercise SECTION A(TRUE/FALSE TYPE QUESTIONS)|5 VideosSOLVED MODEL TEST PAPER-1
ACCURATE PUBLICATION|Exercise SECTION D|14 VideosSOLVED MODEL TEST PAPER-3
ACCURATE PUBLICATION|Exercise SECTION D|12 Videos
Similar Questions
Explore conceptually related problems
ACCURATE PUBLICATION-SOLVED MODEL TEST PAPER-2-SECTION B
- Zn and Cd are not normally considered as transition metals. Why ?
Text Solution
|
- What is the difference between .co-ordination compounds and-Double sal...
Text Solution
|
- Give IUPAC name:
Text Solution
|
- Give IUPAC name:
Text Solution
|
- Benzene and toluene form nearly ideal solution . At 313 K the vapou...
Text Solution
|
- At 298 K the vapour pressure of pure benzene C(6) H(6) is 0.256 bar...
Text Solution
|
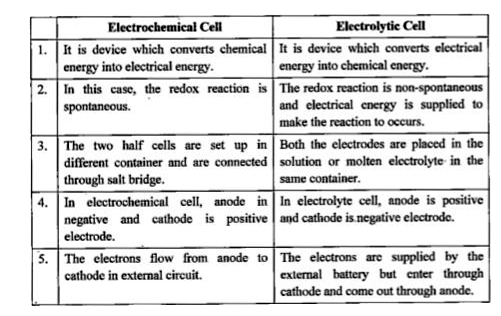
- Write four differences between galvanic (or electrochemical) cell and ...
Text Solution
|
- Why is dioxygen gas but sulphur a solid?
Text Solution
|
- What are the characteristics of the transition elements ?
Text Solution
|
- Write the difference between molecularity and order of reaction?
Text Solution
|
- What are colligative properties ? Name four such properties.
Text Solution
|
- What are the factors affecting the solubility of gas in liquid ?
Text Solution
|
- A first order reaction is 20% complete in the 10 minutes. Calculate th...
Text Solution
|
- Calculate two third life of first order reaction having K = 5.48 xx 10...
Text Solution
|
- Account for the following: Why the acid strengths of halogen acids inc...
Text Solution
|
- Why does fluoriue show anomalous behaviour in its group ?
Text Solution
|