Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ACCURATE PUBLICATION-SOLVED MODEL TEST PAPER-3-SECTION D
- Explain the following reactions: Balz Schiemann reaction.
Text Solution
|
- Explain the following reaction : Sulphonation of Haloarenes
Text Solution
|
- Explain the following reaction : Sandmeyer's reaction
Text Solution
|
- Explain the following reaction : Finckelstein reaction
Text Solution
|
- Write the following reaction: Wurtz Fittig Reaction
Text Solution
|
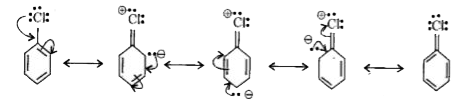
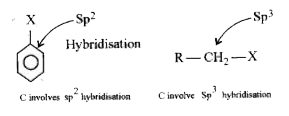
- Why aryl halide(haloarenes) are less reactive than alkyl halide(haloal...
Text Solution
|
- What are ambident nucleophiles? Explain with an example ?
Text Solution
|
- Most of transition metals show variable oxidation states. Explain
Text Solution
|
- Explain why Cu(I) is diamagnetic while Cu(II) is paramagnetic in natur...
Text Solution
|
- Transition metals have high melting and boiling points. Why ?
Text Solution
|
- Define Lanthanide Contraction.
Text Solution
|
- What are the main consequences of lanthanoid contraction ?
Text Solution
|