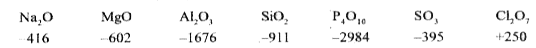A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
BRILLIANT PUBLICATION|Exercise QUESTIONS (LEVEL-III ( Statement Type ))|5 VideosCHEMICAL THERMODYNAMICS
BRILLIANT PUBLICATION|Exercise LEVEL-III|52 VideosENVIRONMENTAL CHEMISTRY
BRILLIANT PUBLICATION|Exercise QUESTIONS LEVEL-II (ASSERTION-REASON TYPE)|10 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES-QUESTIONS (LEVEL-III ( Linked Comprehension Type ))
- The heats of formation (Delta Hf^@) of the oxides of the third period,...
Text Solution
|
- The heats of formation (Delta Hf^@) of the oxides of the third period,...
Text Solution
|
- Ionization energies of five elements, are given below: Which ...
Text Solution
|
- Ionization energies of five elements, are given below: Which t...
Text Solution
|
- Ionization energies of five elements, are given below: The ele...
Text Solution
|
- Ionization energies of five elements, are given below: Most re...
Text Solution
|
- Ionization energies of five elements, are given below: If B r...
Text Solution
|
- Ionization energies of five elements, are given below: Which o...
Text Solution
|
- Identify the least stable ion amongst the following:
Text Solution
|
- The first ionization energy of Na, Mg, Al and Si are in the order of:
Text Solution
|
- Which one of the following statements is incorrect in relation to ioni...
Text Solution
|
- Considering the elements F, Cl, O and S, the correct order of their el...
Text Solution
|