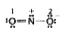A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING
BRILLIANT PUBLICATION|Exercise LEVEL-II (ASSERTION-REASON TYPE)|20 VideosCHEMICAL BONDING
BRILLIANT PUBLICATION|Exercise QUESTIONS|6 VideosCHEMICAL BONDING
BRILLIANT PUBLICATION|Exercise LEVEL-I|120 VideosCHEMICAL AND IONIC EQUILIBRIUM
BRILLIANT PUBLICATION|Exercise Level - III (Linked Comprehension Type Questions)|1 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
BRILLIANT PUBLICATION|Exercise QUESTION (LEVEL -ll)|42 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-CHEMICAL BONDING-LEVEL-II
- Which of the following compounds contains the maximum number of lone p...
Text Solution
|
- Which of the following molecules contains the maximum S-O bond length?...
Text Solution
|
- What are the formal charges of the atoms in the nitrite ion in the ord...
Text Solution
|
- Molecule AB has a bond length of 1.67 Å and a dipole moment of 0.38 D....
Text Solution
|
- Which of the following is arranged in order of increasing dipole momen...
Text Solution
|
- Stability of the species Li2, Li2^(-) and Li2^(+) increases in the ord...
Text Solution
|
- The bond order in NO is 2.5 whereas that in NO^(+) is 3. Which of the ...
Text Solution
|
- Which of the following molecules has the greatest number of lone pairs...
Text Solution
|
- The hybridization of N atoms in NO3^(-), NO2^(+) and NH4^(+) are respe...
Text Solution
|
- The number and type of bonds in C2^(2-) ion in CaC2 are:
Text Solution
|
- Shapes of certain interhalogen compounds are stated below. Which one o...
Text Solution
|
- In which of the following chemical change, the hybridization of the ce...
Text Solution
|
- In which of the following ionization processes, the bond order has inc...
Text Solution
|
- The bond dissociation energy of B-F in BF3 is 646 kJ mol^(-1), whereas...
Text Solution
|
- Among the following the paramagnetic compound is
Text Solution
|
- The bond energy (in kJ mol^(-1)) of C-C single bond is, approximately,
Text Solution
|
- Assuming that Hund's rule is violated, the bond order and magnetic nat...
Text Solution
|
- According MO theory, : O2^(+) is paramagnetic and bond order greate...
Text Solution
|
- The species having bond order different from that in CO is
Text Solution
|
- The "charge"//"size" ratio of a cation determines its polarizing power...
Text Solution
|