Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BETTER CHOICE PUBLICATION-HALOALKANES AND HALOARENES-QUESTIONS
- Arrange each set of compounds in order of increasing boiling points. ...
Text Solution
|
- Haloarenes are insoluble in water but soluble in benzene. Explain.
Text Solution
|
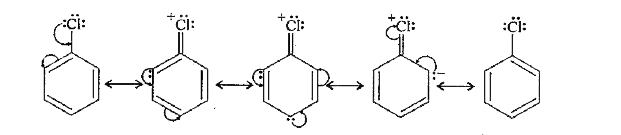
- The dipole moment of chlorobenzene is lower than that of cyclohexyl ch...
Text Solution
|
- Alkyl halides though polar, are immiscible with water, why ?
Text Solution
|
- Define Optical activity.
Text Solution
|
- Explain the term dextrorotatory and laevorotatory.
Text Solution
|
- State and explain the terms enantiomers and enantiomerism. How are the...
Text Solution
|
- What is optical isomerism? What is the necessary and sufficient condit...
Text Solution
|
- Give different Enantiomers of Butanol-2.
Text Solution
|
- Give different Enantiomers of 2 chlorobutane.
Text Solution
|
- Give different Enantiomers of 3-methyl hexane.
Text Solution
|
- State and explain the term diastereoisomers and mesomers.
Text Solution
|
- What is racemisation ?
Text Solution
|
- Define asymmetric synthesis.
Text Solution
|
- Point out the difference between : Chirality and chiral centre (or c...
Text Solution
|
- Point out the difference between : Enantiomers and diastereomers.
Text Solution
|
- Differentiate between recemic mixture and meso compound .
Text Solution
|
- Define inversion.
Text Solution
|
- Define retention.
Text Solution
|
- The p-isomer of dichlorobenzene has higher melting point than O-and M-...
Text Solution
|