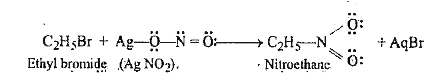Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BETTER CHOICE PUBLICATION-HALOALKANES AND HALOARENES-QUESTIONS
- The treatment of alkyl halides with KNO2 leads of the formation of alk...
Text Solution
|
- Alkyl halides react with AgNO2 to give R-NO2not R-ONO.Why?
Text Solution
|
- The treatment of alkyl halides with alcoholic KOH leads to the formati...
Text Solution
|
- Alkyl halides react with AgNO2 to give R-NO2not R-ONO.Why?
Text Solution
|
- Rearrange the compounds of each of the following sets in order of reac...
Text Solution
|
- Allyl choride is more reactive than r-propyl chloride towards nucleoph...
Text Solution
|
- Haloalkanes react with potassium cyanide (KCN) to give alkyl cyanide, ...
Text Solution
|
- Although chlorine is an electron withdrawing group, yet it is ortho-, ...
Text Solution
|
- How will you convert the following : Isopropylbromide to Propene
Text Solution
|
- How will you convert the following : Bromoethane to iodoethane.
Text Solution
|
- How will you convest the following: Chlorobencene to Aniline
Text Solution
|
- How will you convest the following: Aniline to Fluorobencene
Text Solution
|
- How will you convert. Anline into chlorobenzene.
Text Solution
|
- Complete the following reaction : CH3 CH2 Br + Ag CN to
Text Solution
|
- How will you convert Ethylbromide to Ethylisocyanide.
Text Solution
|
- Give chemical test to distinguish between following pair of compounds.
Text Solution
|
- Give chemical test to distinguish between following pair of compounds....
Text Solution
|
- Give chemical test to distinguish between following pair of compounds....
Text Solution
|
- How will you differentiate between S(N^1) and S(N^2) reaction mechanis...
Text Solution
|
- What are freons ?
Text Solution
|