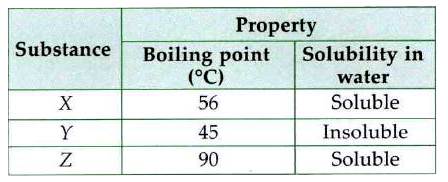
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MTG IIT JEE FOUNDATION-IS MATTER AROUND US PURE ?-OLYMPIAD/ HOTS CORNER
- Three students Ankit, Dinesh and Manoj were given three unknown substa...
Text Solution
|
- A mixture of iodine and carbon tetrachloride is
Text Solution
|
- Solubility of KNO3
Text Solution
|
- Four samples P, Q, R and S were analysed using paper chromatography to...
Text Solution
|
- When two liquids in a mixture differ by their boiling points. Which of...
Text Solution
|
- A special technique used nowadays for separation and identification of...
Text Solution
|
- The path of light gets illuminated when passed through the
Text Solution
|
- The colloidal solution among the following is
Text Solution
|
- Milk of magnesia is an example of which type of colloid?
Text Solution
|
- The given diagram represents an activity to separate a mixture of two ...
Text Solution
|
- What will be the mass by mass percentage of a solution containing 30 g...
Text Solution
|
- Which of the following shows the Tyndall effect?
Text Solution
|
- Which of the following statements are correct about properties of coll...
Text Solution
|
- Ms Diksha, a science teacher gave different substances to three studen...
Text Solution
|
- Read the given passage and fill in the blanks by choosing an appropria...
Text Solution
|
- Which of the following statements is correct in context to Tyndall eff...
Text Solution
|
- Choose the correct option about cheese.
Text Solution
|
- What type of colloidal system is fog?
Text Solution
|
- Match the Column-I with Column-II and choose the correct option using ...
Text Solution
|
- Match the column I with column II and select the correct option from t...
Text Solution
|