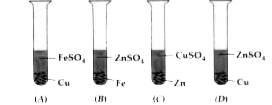Text Solution
Verified by Experts
Topper's Solved these Questions
MATERIALS : METALS AND NON-METALS
MTG IIT JEE FOUNDATION|Exercise NCERT Section|11 VideosMATERIALS : METALS AND NON-METALS
MTG IIT JEE FOUNDATION|Exercise EXERCISE MCQ.s (Level-1)|30 VideosMATERIALS : METALS AND NON-METALS
MTG IIT JEE FOUNDATION|Exercise Olympiad/HOTS Corner|20 VideosFOOTSTEPS towards(CBSE Board)
MTG IIT JEE FOUNDATION|Exercise SECTION - D|14 VideosMATTER : ELEMENTS COMPOUNDS AND MIXTURES
MTG IIT JEE FOUNDATION|Exercise EXERCISE (INTEGER/NUMERICAL VALUE )|5 Videos
Similar Questions
Explore conceptually related problems
MTG IIT JEE FOUNDATION-MATERIALS : METALS AND NON-METALS-Solved Examples
- In the following four test tubes, some metals are in contact with cert...
Text Solution
|
- A silver spoon is kept immersed in an aqueous solution of copper sulph...
Text Solution
|
- A solution of copper sulphate was stored in an iron container. After a...
Text Solution
|
- What happens when samples of metals and non-metals are mixed with acid...
Text Solution
|
- Compare metals and non-metals on the basis of chemical properties.
Text Solution
|
- Name the metals present in the bases and non-metals present in the aci...
Text Solution
|
- Which of the following reaction cannot take place ? Justify your answe...
Text Solution
|
- Complete and balance the chemical equation for the following reactions...
Text Solution
|
- Explain reaction of sodium, magnesium and iron with water.
Text Solution
|
- (i) What is an alloy ? (ii) A light and strong alloy is required for...
Text Solution
|
- Define the terms : Galvanized iron and passive iron.
Text Solution
|
- Metal M occurs in earth's crust as its oxide M(2)O(3). An alloy of thi...
Text Solution
|
- Are metals a renewable resources ? If not, can they be recycled ?
Text Solution
|
- What is malleability ? Name two most malleable metals.
Text Solution
|
- Explain the reaction of sodium and water with the help of an activity.
Text Solution
|