Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ACCURATE PUBLICATION-D & F BLOCK ELEMENTS -2 OR 5 MARKS QUESTIONS
- What is atomicity ? Explain with two examples.
Text Solution
|
- What is the atomicity of the following : oxygen
Text Solution
|
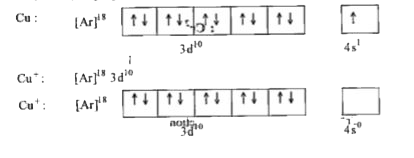
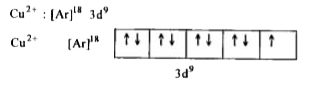
- Explain why Cu(I) is diamagnetic while Cu(II) is paramagnetic in natur...
Text Solution
|
- Transition metals have high melting points and boiling points. Why?
Text Solution
|
- What is the atomicity of the following : ozone
Text Solution
|
- What is the atomicity of the following : neon
Text Solution
|
- What is the atomicity of the following : phosphorous
Text Solution
|
- What is the atomicity of the following : sulphur
Text Solution
|
- What is the atomicity of the following : sodium
Text Solution
|
- What are lanthanoids ?
Text Solution
|
- Why are Lanthanides called inner transition metals.
Text Solution
|
- What are different oxidation states exhibited by lanthanoids ?
Text Solution
|
- What is lanthanoid contraction ?
Text Solution
|
- Why is La(OH)3 more basic than Lu(OH)3 ?
Text Solution
|
- Why Zr and Hf exhibit similar properties ?
Text Solution
|
- Write the formulas of ammonia . Also name the elements presents in the...
Text Solution
|
- Write the formulas of methane . Also name the elements presents in th...
Text Solution
|
- Write the formula of sulphur dioxide . Also name the elements presents...
Text Solution
|
- Write the formula of ethanol . Also name the elements presents in them...
Text Solution
|
- Write the formula of calcium carbonate . Also name the elements presen...
Text Solution
|