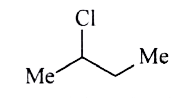
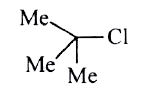
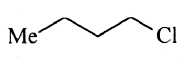
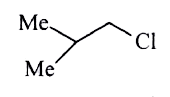
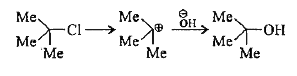A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ISOMERISM AND REACTION MECHANISM
BRILLIANT PUBLICATION|Exercise LEVEL - III (Multiple choice answer type)|15 VideosISOMERISM AND REACTION MECHANISM
BRILLIANT PUBLICATION|Exercise LEVEL - III (Numerical Type)|6 VideosISOMERISM AND REACTION MECHANISM
BRILLIANT PUBLICATION|Exercise LEVEL-II |40 VideosHYDROGEN
BRILLIANT PUBLICATION|Exercise LEVEL -III ( Linked Comprehension Type)|8 VideosORGANIC CHEMISTRY : SOME BASIC PRINCIPLES - PART I (NOMENCLATURE)
BRILLIANT PUBLICATION|Exercise LEVEL-III|45 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-ISOMERISM AND REACTION MECHANISM-LEVEL - III
- Equal amount of an RCl(C4H9Cl) is reacted at the same temperature with...
Text Solution
|
- Which of the following statements is correct about the following react...
Text Solution
|
- Which compound in each of the following pairs will react faster in SN2...
Text Solution
|
- Consider thiol anion (RS^(Theta)) and alkoxy anion (RO^(Theta)). Which...
Text Solution
|
- The rate of the reaction, is influenced by the hyperconjugation ...
Text Solution
|
- For the following reactions: (A) CH3 CH2 CH2 Br + KOH to CH3 CH = C...
Text Solution
|
- Which of the following is the least stable carbocation?
Text Solution
|
- Select the correct statement(s) given below: (I) Both - Cl group as...
Text Solution
|