Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
OMEGA PUBLICATION-THE P-BLOCK ELEMENTS-MULTIPLE CHOICE QUESTIONS
- Comment on nature of two S-O bond formed in SO2 molecule. Are the two ...
Text Solution
|
- Bauxite containing chief impurities of oxides of silicon is called
Text Solution
|
- Thermite is a mixture of
Text Solution
|
- The first ionisation energy of silicon is lower than that of
Text Solution
|
- Account for the following: The +2 oxidation state of lead is more stab...
Text Solution
|
- Define catenation?
Text Solution
|
- Silicon hydrides are called
Text Solution
|
- C Cl4 is not hydrolysed but SiCl4 can be hydrolysed with water. Why ?
Text Solution
|
- The formula of dry ice is ………………….. .
Text Solution
|
- Which of the following is an ore of boron?
Text Solution
|
- Compounds of Boron with hydrogen are known as
Text Solution
|
- Diborane has
Text Solution
|
- In B(2)H(6) , B-atom is
Text Solution
|
- Orthoboric acid is
Text Solution
|
- Boron halides are
Text Solution
|
- Ammonia is, in general,
Text Solution
|
- NH(3) can be prepared by
Text Solution
|
- Boron trioxide can be reduced to boron with
Text Solution
|
- In reaction BF(3) + 3LiBH(4) rarr 3LiF+ X, X is
Text Solution
|
- Graphite is a good conductor of electricity because
Text Solution
|
- The laughing gas is
Text Solution
|
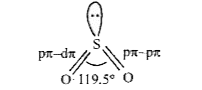 In `SO_(2)` molecule, S is `sp^(2)` hybridised. Two of three `sp^(2)` orbitals forms two o- bonds while third contains lone pair of electrons. S is left with one half filled p-orbital and one half filled d- orbital forming one p`pi`-p`pi` and one p`pi`-d`pi` double bond with oxygen atom. So `SO_(2)` has bent structure having OSO bond angle of 119.5° and.due to resonance both `pi`-bonds are equal (143 pm).
In `SO_(2)` molecule, S is `sp^(2)` hybridised. Two of three `sp^(2)` orbitals forms two o- bonds while third contains lone pair of electrons. S is left with one half filled p-orbital and one half filled d- orbital forming one p`pi`-p`pi` and one p`pi`-d`pi` double bond with oxygen atom. So `SO_(2)` has bent structure having OSO bond angle of 119.5° and.due to resonance both `pi`-bonds are equal (143 pm).