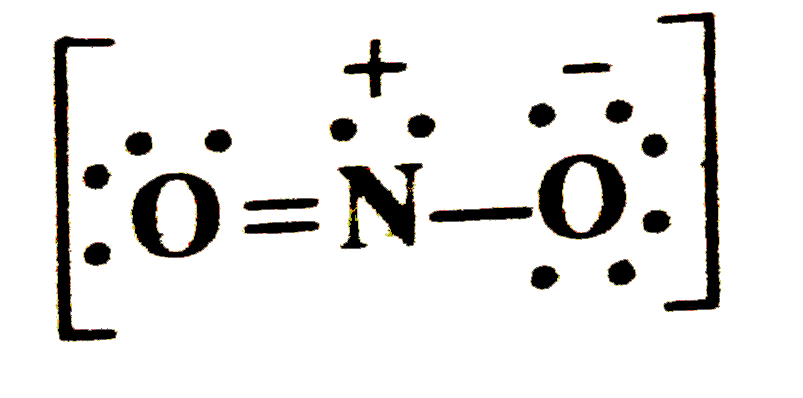Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
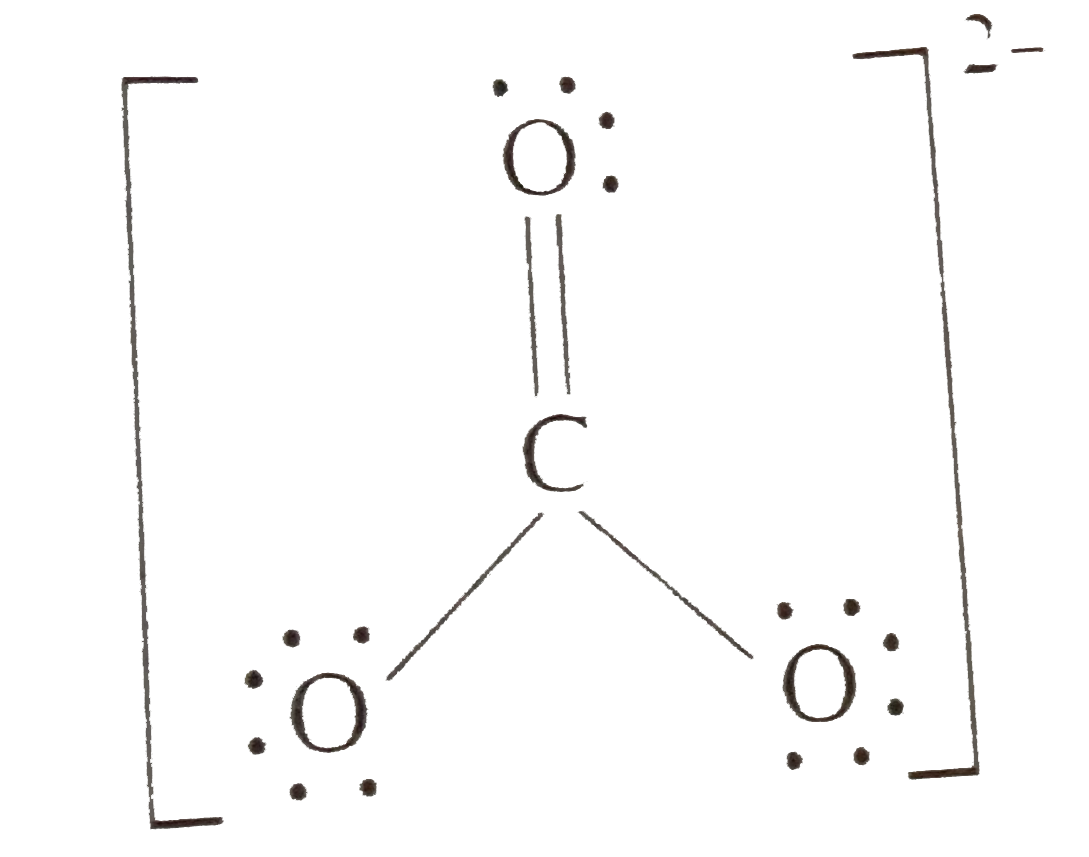
- Write the formal charges on atoms in (i) and carbonate ion (ii) nitrit...
Text Solution
|
- Calculate the formal charge on each atoms in nitrite ion.
Text Solution
|
- The formal charge of the O-atom in the ion [: ddotN = O:] is
Text Solution
|
- Write the formal charge on atoms in (i) carbonate ion (ii) nitrite ion
Text Solution
|
- Calculate the formal charge on C-atom in carbonate ion.
Text Solution
|
- In NO(3)^(-) ion the formal charge on the oxygen atom of N-O bond is
Text Solution
|
- Write the formal charges of the atoms in (i) hydroxide ion (ii) carbon...
Text Solution
|
- In bisulphate ion the formal charge on sulphur atom is
Text Solution
|
- In PO4(3-) ion, the formal charge on the oxygen atom of P-O bonds is
Text Solution
|