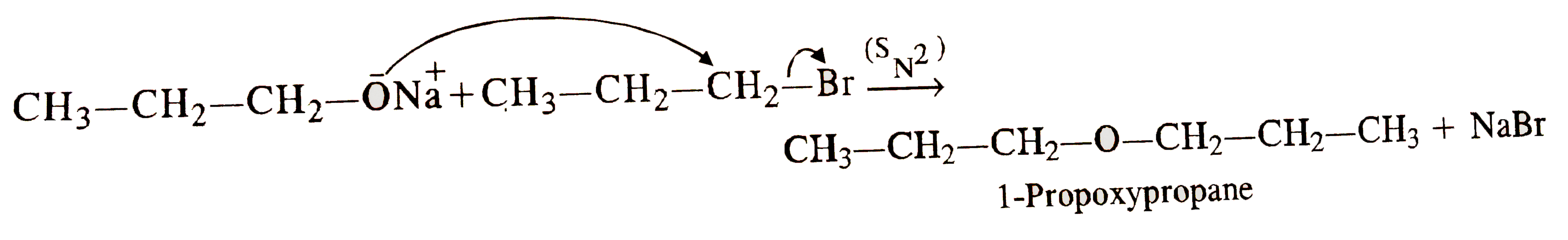Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
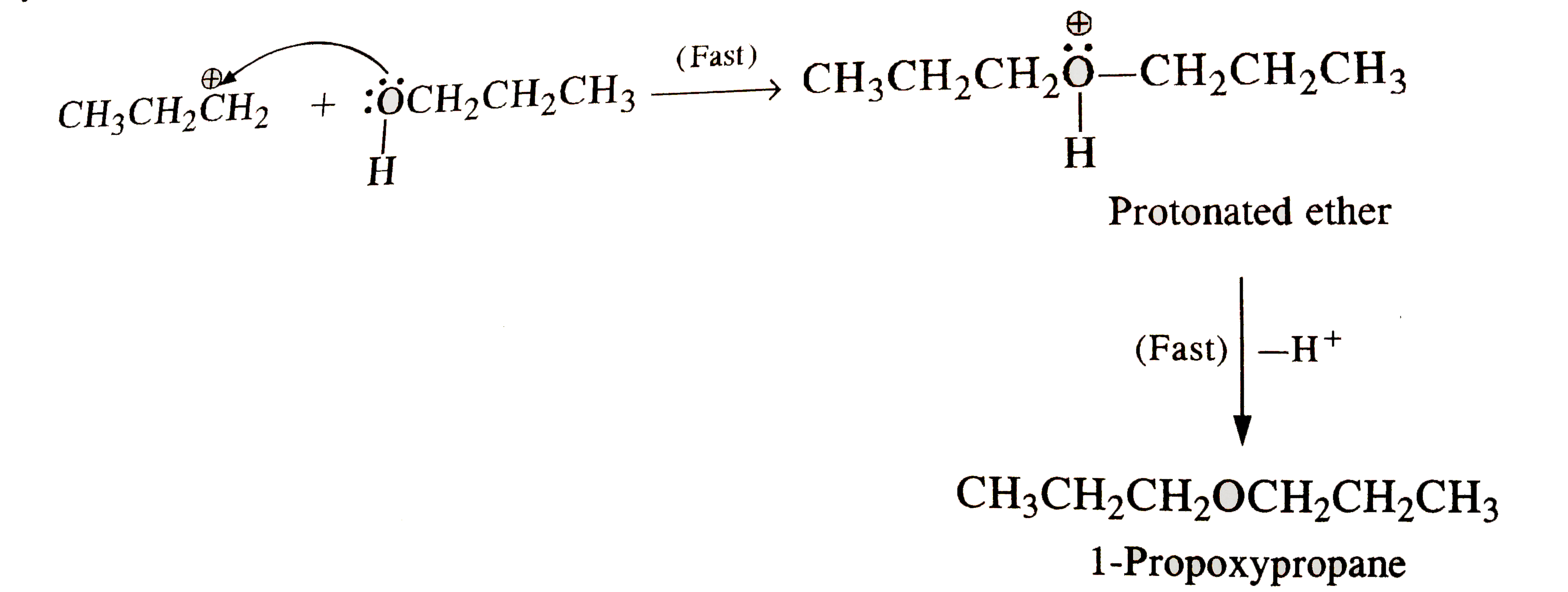
- How is 1-propoxypropane synthesised from propan-1-ol ? Write mechanism...
Text Solution
|
- Synthesise the following : a. Butene to butanol and butan-2-ol 1-...
Text Solution
|
- प्रोपेन-1-ऑल से 1-प्रोपॉक्सीप्रोपेन को किस प्रकार बनाया जाता है? अभिक्...
Text Solution
|
- प्रोपेन-1-ऑल से 1-प्रोपोक्सीप्रोपेन किस प्रकार प्राप्त करेंगे ? अभिक्र...
Text Solution
|
- How is 1-propoxyropane synthesized from propan-1-ol ? Write mechanism ...
Text Solution
|
- प्रोपेन-1 ऑल से 1-प्रोपाक्सीप्रोपेन किस प्रकार बनायेंगे, इस अभिक्रिया ...
Text Solution
|
- How is 1-propoxyproapane synthesised from propan-1-ol ? Write mechanis...
Text Solution
|
- Propan - 1 - ol एवं Propan - 2 - ol को कैसे विभेद करेंगे
Text Solution
|
- Propan- 1 -ol and propan- 2 -ol are ......... isomers.
Text Solution
|