A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MS CHOUHAN-CARBENE AND NITRENE -LEVEL-2 (Comprehension)
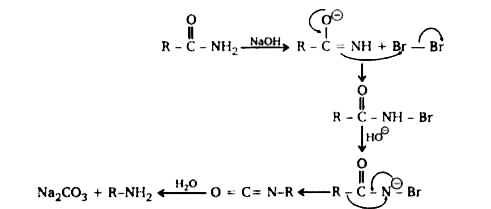
- Hoffmann bromamide reaction involves conversion of a carboxylic acid a...
Text Solution
|
- Hoffmann bromamide reaction involves conversion of a carboxylic acid a...
Text Solution
|
- Hoffmann bromamide reaction involves conversion of a carboxylic acid a...
Text Solution
|
- Hoffmann bromamide reaction involves conversion of a carboxylic acid a...
Text Solution
|
- Given is mechanism of Beckmann rearrangement. Rate determining...
Text Solution
|
- Given is mechanism of Beckmann rearrangement. On treatment ...
Text Solution
|
- Given is mechanism of Beckmann rearrangement. Which of the fol...
Text Solution
|
- Given is mechanism of Beckmann rearrangement. Product (A) o...
Text Solution
|
- Consider the given reaction for preparation of alkyne. (Fritsch reacti...
Text Solution
|
- Consider the given reaction for preparation of alkyne. (Fritsch reacti...
Text Solution
|
- Consider the given reaction for preparation of alkyne. (Fritsch reacti...
Text Solution
|
- Consider the given reaction for preparation of alkyne. (Fritsch reacti...
Text Solution
|
- Wolff rearrangement When alpha-Diazoketones are photo-irradiated or...
Text Solution
|
- Wolff rearrangement When alpha-Diazoketones are photo-irradiated or...
Text Solution
|
- Wolff rearrangement When alpha-Diazoketones are photo-irradiated or...
Text Solution
|
- Wolff rearrangement When alpha-Diazoketones are photo-irradiated or...
Text Solution
|
- Wolff rearrangement When alpha-Diazoketones are photo-irradiated or...
Text Solution
|
- Wolff rearrangement When alpha-Diazoketones are photo-irradiated or...
Text Solution
|
- Wolff rearrangement When alpha-Diazoketones are photo-irradiated or...
Text Solution
|