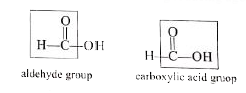Text Solution
Verified by Experts
Topper's Solved these Questions
CARBONYL COMPOUNDS AND CARBOXYLIC ACIDS
PREMIERS PUBLISHERS|Exercise OTHER IMORTENT QUESTIONS & ANSWERS ( CHOOSE THE CORRECT ANSWER.)|29 VideosBIOMOLECULES
PREMIERS PUBLISHERS|Exercise OTHER IMPORTANT QUESTIONS& ANSWERS (Answer the following questions)|66 VideosCHEMICAL KINETICS
PREMIERS PUBLISHERS|Exercise OTHER IMPORTANT QUESTIONS & ANSWERS (II. Answer the following questions.)|52 Videos
Similar Questions
Explore conceptually related problems
PREMIERS PUBLISHERS-CARBONYL COMPOUNDS AND CARBOXYLIC ACIDS-OTHER IMORTENT QUESTIONS & ANSWERS (ANSWER THE FOLLOWING QUESTIONS)
- Explain why the 'COOH' group in benzoic acid in meta directing.
Text Solution
|
- How are the following compounds prepared from benzoic acid ? (i) m -...
Text Solution
|
- Formic acid is a reducing agent. Substantriate this statement with exa...
Text Solution
|
- Give the tests for carboxylic acid group in an organic compound.
Text Solution
|
- Give a brief accout of acidity of carboxylic acids.
Text Solution
|
- Briefly discuss the effect substituents on the acidity of carboxylic a...
Text Solution
|
- Fluorine is more electroegative than chlorine even their p - fluoro be...
Text Solution
|
- Explian why p- nitrobenzoic acid has a higher Ka value than benzoic ac...
Text Solution
|
- Give the IUPAC names of the following compounds. (i) PhCH2CH2COOH (i...
Text Solution
|
- Explain the mechanism ffor the reaction.
Text Solution
|
- Give the uses of (i) formic acid (ii) acetic acid (iii) benzoic acid (...
Text Solution
|
- A compound with moelcular formula, C4H10C3 on acetylation with acetic ...
Text Solution
|
- Explain (i) Perkins'reaction (ii) Knoevenagal reaction.
Text Solution
|
- Give names of reagent which bring about the following conversions. (i...
Text Solution
|
- Bring out the following conversions. (i) Benzyl alcohol to phenyl et...
Text Solution
|
- Identigy A - E in the following reactions :
Text Solution
|
- Write a short note on electrophilic substitution reaction of benzaldeh...
Text Solution
|
- Which acid of each pair shown here would you expect to be stronger ? ...
Text Solution
|
- Identify A to E in the following reactions :
Text Solution
|
- How will you bring about the following conversions in not more than tw...
Text Solution
|