A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ACIDS, BASES AND SALTS
OSWAL PUBLICATION|Exercise Self Assessment - 1 (I. Objective Type Questions) A. Multiple Choice Questions|3 VideosACIDS, BASES AND SALTS
OSWAL PUBLICATION|Exercise Self Assessment - 1 (I. Objective Type Questions) B. Passage / Table Based Questions|4 VideosACIDS, BASES AND SALTS
OSWAL PUBLICATION|Exercise ASSERTION AND REASON BASED MCQs |8 VideosCARBON AND ITS COMPOUNDS
OSWAL PUBLICATION|Exercise CREATING BASED QUESTIONS|23 Videos
Similar Questions
Explore conceptually related problems
OSWAL PUBLICATION-ACIDS, BASES AND SALTS-CASE-BASED MCQs
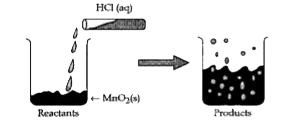
- Read the following and answer the questions. The reaction between Mn...
Text Solution
|
- The reaction between MnO2 with HCl is depicted in the following diagr...
Text Solution
|
- Read the following and answer the questions. The reaction between Mn...
Text Solution
|
- The reaction between MnO2 with HCl is depicted in the following diagr...
Text Solution
|
- The reaction between MnO2 with HCl is depicted in the following diagr...
Text Solution
|
- Frothing in Yamuna: The primary reason behind the formation of the ...
Text Solution
|
- Frothing in Yamuna: The primary reason behind the formation of the ...
Text Solution
|
- Frothing in Yamuna: The primary reason behind the formation of the ...
Text Solution
|
- Frothing in Yamuna: The primary reason behind the formation of the ...
Text Solution
|
- Frothing in Yamuna: The primary reason behind the formation of the ...
Text Solution
|
- Read the following and answer the questions. Study the given table...
Text Solution
|
- Read the following and answer the questions. Study the given table...
Text Solution
|
- Read the following and answer the questions. Study the given table...
Text Solution
|
- Read the following and answer the questions. Study the given table...
Text Solution
|
- Suhana takes three beakers, A, B and C filled with aqueous solutions...
Text Solution
|
- Suhana takes three beakers, A, B and C filled with aqueous solutions...
Text Solution
|
- Suhana takes three beakers, A, B and C filled with aqueous solutions...
Text Solution
|
- Suhana takes three beakers, A, B and C filled with aqueous solutions...
Text Solution
|
- Study the given experimental set-up and answer the following question...
Text Solution
|
- Study the given experimental set-up and answer the following question...
Text Solution
|