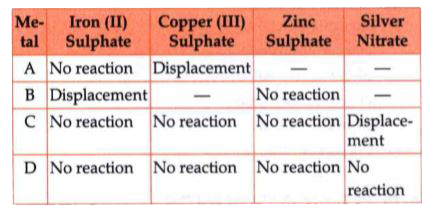
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
NEW CHAPTERS AND QUESTIONS BASED ON LATEST TYPOLOGIES INTRODUCED BY CBSE FOR 2021-22 EXAMINATION
OSWAL PUBLICATION|Exercise CARBON AND ITS COMPOUNDS (SELF ASSESSMENT) (OBJECTIVE TYPE QUESTIONS) (A. Multiple Choice Questions)|3 VideosNEW CHAPTERS AND QUESTIONS BASED ON LATEST TYPOLOGIES INTRODUCED BY CBSE FOR 2021-22 EXAMINATION
OSWAL PUBLICATION|Exercise CARBON AND ITS COMPOUNDS (SELF ASSESSMENT) (OBJECTIVE TYPE QUESTIONS) (B. Assertion and Reason Type Questions )|2 VideosNEW CHAPTERS AND QUESTIONS BASED ON LATEST TYPOLOGIES INTRODUCED BY CBSE FOR 2021-22 EXAMINATION
OSWAL PUBLICATION|Exercise METALS AND NON- METALS (SELF ASSESSMENT) (LONG ANSWER TYPE QUESTIONS)|1 VideosMETALS AND NON-METALS
OSWAL PUBLICATION|Exercise Board corner(Long answer type questions)|20 VideosNTSE 2019 - 20
OSWAL PUBLICATION|Exercise (CHEMISTRY) ATOMS AND MOLECULES (STAGE - 2)|1 Videos
Similar Questions
Explore conceptually related problems
OSWAL PUBLICATION-NEW CHAPTERS AND QUESTIONS BASED ON LATEST TYPOLOGIES INTRODUCED BY CBSE FOR 2021-22 EXAMINATION -METALS AND NON- METALS (VISUAL CASE - BASED QUESTIONS)
- During an experiment, Rohan took the samples of four metals A, B, C an...
Text Solution
|
- During an experiment, Rohan took the samples of four metals A, B, C an...
Text Solution
|
- During an experiment, Rohan took the samples of four metals A, B, C an...
Text Solution
|
- During an experiment, Rohan took the samples of four metals A, B, C an...
Text Solution
|
- During an experiment, Rohan took the samples of four metals A, B, C an...
Text Solution
|
- During extraction of metals, electrolytic refining is used to obtain p...
Text Solution
|
- During extraction of metals, electrolytic refining is used to obtain p...
Text Solution
|
- During extraction of metals, electrolytic refining is used to obtain p...
Text Solution
|
- During extraction of metals, electrolytic refining is used to obtain p...
Text Solution
|
- During extraction of metals, electrolytic refining is used to obtain p...
Text Solution
|
- Rajat knows that in a thermite reaction, a compound of iron reacts wit...
Text Solution
|
- Rajat knows that in a thermite reaction, a compound of iron reacts wit...
Text Solution
|
- Rajat knows that in a thermite reaction, a compound of iron reacts wit...
Text Solution
|
- Rajat knows that in a thermite reaction, a compound of iron reacts wit...
Text Solution
|
- Rajat knows that in a thermite reaction, a compound of iron reacts wit...
Text Solution
|