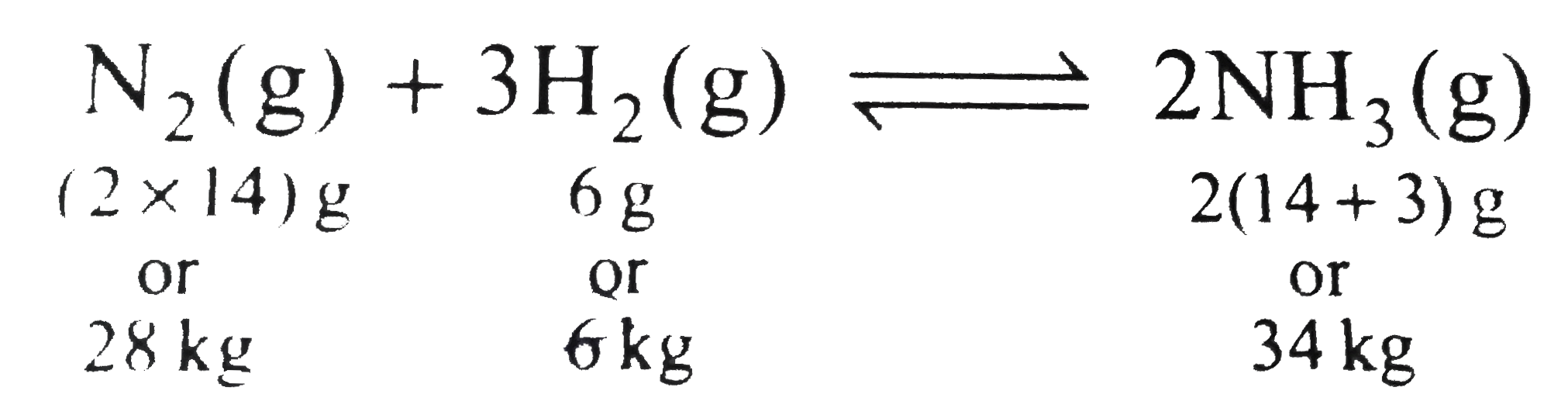Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- 50.0 kg of N(2) (g) and 10.0 kg of H(2) (g) are mixed, to produce NH(3...
Text Solution
|
- 28 g of N(2) and 6 g of H(2) were mixed. At equilibrium 17 g NH(3) was...
Text Solution
|
- 50.0 kg of N(2)(g) and 10.0 kg of H(2)(g) are mixed to produce NH(3)(g...
Text Solution
|
- 20.0 kg of N(2(g)) and 3.0 kg of H(2(g)) are mixed to produce NH(3(g))...
Text Solution
|
- 20.0 kg of N(2)(g) and 3.0 kg of H(2)(g) are mixed to produce NH(3)(g)...
Text Solution
|
- 50.00 kg N(2) (g) और 10.00 kg H(2) (g) को NH(3)(g) बनाने के लिए म...
Text Solution
|
- For the reactions, N(2(g))+3H(2(g))hArr2NH(3(g)). At 400 K, K(p)=41 at...
Text Solution
|
- 20.0 kg of N(2)(g) and 3.0 kg of H(2)(g) are mixed to produce NH(3)(g)...
Text Solution
|
- In Haber's process 50.0g of N(2) [g] and 10.0 g of H(2) [g] are mixed ...
Text Solution
|