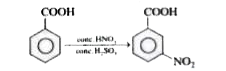
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Explain the orientation of-COOH group, when present on benzene ring.
Text Solution
|
- The -COOH group in benzne ring is
Text Solution
|
- Why does presence of a nifro group make the benzene ring less reactive...
Text Solution
|
- Of the following four groups are m- directing when present on a benzen...
Text Solution
|
- Explain OH group attached to benzene ring activates it towards electro...
Text Solution
|
- Explain why it is very difficult to introduce the third -NO2 group in...
Text Solution
|
- Explain the theory of orientation of substitution in benzene ring with...
Text Solution
|
- बेंजीन में उपस्थित एक इलेक्ट्रॉन दानी समूह, आने वाले इलेक्ट्रॉन - स्ने...
Text Solution
|
- How to insert the given groups into the benzene ring: —COOH
Text Solution
|